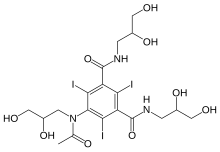
Iohexol
 |
|
| Clinical data | |
|---|---|
| Trade names | Omnipaque, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration |
intrathecal, intravascular, by mouth, intracavital, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Low |
| Metabolism | Nil |
| Biological half-life | Variable |
| Excretion | Kidney, unchanged |
| Identifiers | |
|
|
| Synonyms | 5-[N-(2,3-Dihydroxypropyl)acetamido]-2,4,6-triiodo-N,N'-bis(2,3-dihydroxypropyl)isophthalamide |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.060.130 |
| Chemical and physical data | |
| Formula | C19H26I3N3O9 |
| Molar mass | 821.138 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Iohexol, sold under the trade names Omnipaque among others, is a contrast agent used during X-rays. This includes when visualizing arteries, veins, ventricles of the brain, the urinary system, and joints, as well as during computer tomography (CT scan). It is given by mouth, injection into a vein, or into a body cavity.
Side effects include vomiting, skin flushing, headache, itchiness, kidney problems, and low blood pressure. Less commonly allergic reactions or seizures may occur. It should not be used by those who have a iodine allergy. Use in the later part of pregnancy may cause hypothyroidism in the baby. Iohexol is an iodinated non-ionic radiocontrast agent. It is in the low osmolar family.
Iohexol was approved for medical use in 1985. It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system. The wholesale cost in the developing world is about 10.99 USD per 50 ml vial. In the United States a dose costs 50 to 100 USD.
...
Wikipedia
