Iodixanol
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Multum Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | Negligible |
| Metabolism | Excreted unchanged |
| Biological half-life | 2.1 hours |
| Excretion | Renal |
| Identifiers | |
|
|
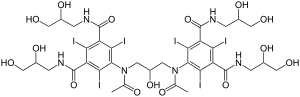
| Synonyms | 5-[acetyl-[3-[acetyl-[3,5-bis(2,3-dihydroxypropylcarbamoyl)-2,4,6-triiodo-phenyl]amino]-2-hydroxy-propyl]amino]-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-benzene-1,3-dicarboxamide |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.124.306 |
| Chemical and physical data | |
| Formula | C35H44I6N6O15 |
| Molar mass | 1550.191 |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Iodixanol is a contrast agent, sold under the trade name Visipaque; it is also sold as a density gradient medium under the name OptiPrep. Visipaque is commonly used as a contrast agent during coronary angiography. It is the only iso-osmolar contrast agent, with an osmolality of 290 mOsm/kg H2O, the same as blood. It is sold in 2 main concentrations 270 mgI/ml and 320 mgI/ml - hence the name Visipaque 270 or 320. It is sold in single dose units and a large 500ml plastic bottle for multi-dose dispensing
It was originally made by Nycomed, which then merged with Amersham which finally was bought by Jeff Immelt to become GE Healthcare. The majority of Visipaque is made in Cork, Ireland, with additional plants in China and Oslo. All Iodixanol bulk powder is made in Norway by Axis-Shield and shipped to the various plants for final manufacture (distributed in the United States by Cosmo Bio USA).
A very similar product also manufactured by GE Healthcare is Omnipaque (Iohexol as the main drug substance).
...
Wikipedia
