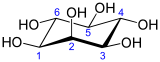
Inositol
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
(1R,2R,3S,4S,5R,6S)-cyclohexane-1,2,3,4,5,6-hexol
|
|
| Other names
cis-1,2,3,5-trans-4,6-Cyclohexanehexol , Cyclohexanehexol,
Mouse antialopecia factor, Nucite, Phaseomannite, Phaseomannitol, Rat antispectacled eye factor, and Scyllite (for the structural isomer scyllo-Inositol) |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g/mol |
| Density | 1.752 g/cm3 |
| Melting point | 225 to 227 °C (437 to 441 °F; 498 to 500 K) |
| Pharmacology | |
| A11HA07 (WHO) | |
| Hazards | |
| NFPA 704 | |
| Flash point | 143 °C (289 °F; 416 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Inositol or cyclohexane-1,2,3,4,5,6-hexol is a chemical compound with formula C6H12O6 or (-CHOH-)6, a six-fold alcohol (polyol) of cyclohexane. It exists in nine possible stereoisomers, of which the most prominent form, widely occurring in nature, is cis-1,2,3,5-trans-4,6-cyclohexanehexol, or myo-inositol (former names meso-inositol or i-inositol). Inositol is a sugar alcohol. Its taste has been assayed at half the sweetness of table sugar (sucrose).
myo-Inositol plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells, the various inositol phosphates. In addition, inositol serves as an important component of the structural lipids phosphatidylinositol (PI) and its various phosphates, the (PIP) lipids.
Inositol or its phosphates and associated lipids are found in many foods, in particular fruit, especially cantaloupe and oranges. In plants, the hexaphosphate of inositol, phytic acid or its salts, the phytates, are found. These serve as phosphate stores in the seed. Phytic acid occurs also in cereals with high bran content and also nuts and beans. Yet, inositol, when present as phytate, is not directly bioavailable to humans in the diet, since it is not digestible. Some food preparation techniques partly break down phytates to change this. Inositol as it occurs in certain plant-derived substances such as lecithins, however, is well-absorbed and relatively bioavailable.
...
Wikipedia

