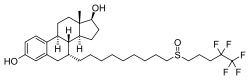
ICI-182,780
 |
|
| Clinical data | |
|---|---|
| Trade names | Faslodex, others |
| Synonyms | ICI-182780; ZD-182780; ZD-9238; 7α-[9-[(4,4,5,5,5-Pentafluoropentyl)-sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17β-diol |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration |
Intramuscular injection |
| Drug class | Antiestrogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 99% |
| Biological half-life | 40 days |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C32H47F5O3S |
| Molar mass | 606.78 g·mol−1 |
| 3D model (JSmol) | |
|
|
|
|
|
|
|
Fulvestrant, sold under the brand name Faslodex among others, is a medication used to treat hormone receptor (HR)-positive metastatic breast cancer in postmenopausal women with disease progression as well as HR-positive, HER2-negative advanced breast cancer in combination with palbociclib in women with disease progression after endocrine therapy. It is given by injection into a muscle.
Fulvestrant is a selective estrogen receptor degrader (SERD) and was first-in-class to be approved. It works by binding to the estrogen receptor and destabilizing it, causing the cell's normal protein degradation processes to destroy it.
Fulvestrant was approved for medical use in the United States in 2002.
Fulvestrant is used for the treatment of hormone receptor positive metastatic breast cancer or locally advanced unresectable disease in postmenopausal women; it is given by injection. A 2017 Cochrane review found it is as safe and effective as first line or second line endocrine therapy.
It is also used to treat HR-positive, HER2-negative advanced or metastatic breast cancer in combination with palbociclib in women with disease progression after first-line endocrine therapy.
It should not be used in women with kidney failure or who are pregnant.
Due to the medication having a similar chemical structure to estrogen, it can interact with immunoassays for blood estradiol concentrations and show falsely elevated results. This can improperly lead to discontinuing the treatment.
Very common (occurring in more than 10% of people) adverse effects include nausea, injection site reactions, weakness, and elevated transaminases. Common (between 1% and 10%) adverse effects include urinary tract infections, hypersensitivity reactions, loss of appetite, headache, blood clots in veins, hot flushes, vomiting, diarrhea, elevated bilirubin, rashes, and back pain.
...
Wikipedia
