Hydroxyproline
 |
|
| Names | |
|---|---|
|
IUPAC name
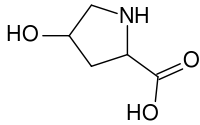
(2S,4R)-4-hydroxypyrrolidine-2-carboxylic acid
|
|
| Identifiers | |
|
51-35-4 |
|
| 3D model (Jmol) | Interactive image |
| ChemSpider |
5605 |
| ECHA InfoCard | 100.000.084 |
| MeSH | Hydroxyproline |
| PubChem | 5810 |
| UNII |
RMB44WO89X |
|
|
|
|
| Properties | |
| C5H9NO3 | |
| Molar mass | 131.13 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
(2S,4R)-4-Hydroxyproline, or L-hydroxyproline (C5H9O3N), is a common non-proteinogenic amino acid, abbreviated as Hyp, e.g., in Protein Data Bank.
In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline.
Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom.
Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl hydroxylase following protein synthesis (as a post-translational modification). The enzyme catalysed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated.
Hydroxyproline is a major component of the protein collagen, comprising roughly 13.5% of mammalian collagen. Hydroxyproline and proline play key roles for collagen stability. They permit the sharp twisting of the collagen helix. In the canonical collagen Xaa-Yaa-Gly triad (where Xaa and Yaa are any amino acid), a proline occupying the Yaa position is hydroxylated to give a Xaa-Hyp-Gly sequence. This modification of the proline residue increases the stability of the collagen triple helix. It was initially proposed that the stabilization was due to water molecules forming a hydrogen bonding network linking the prolyl hydroxyl groups and the main-chain carbonyl groups. It was subsequently shown that the increase in stability is primarily through stereoelectronic effects and that hydration of the hydroxyproline residues provides little or no additional stability. In addition to collagen, the mammalian proteins elastin and argonaute 2 have collagen-like domains in which hydroxyproline is formed. Some snail poisons, conotoxins, contain hydroxyproline, but lack collagen-like sequences.
...
Wikipedia
