Holmium(III) chloride
 |
|
| Names | |
|---|---|
| Other names
Holmium trichloride
Holmiumchlorid |
|
| Identifiers | |
|
|
| ECHA InfoCard | 100.030.339 |
|
PubChem CID
|
|
| UNII | |
| Properties | |
| HoCl3 | |
| Molar mass | 271.289 g/mol |
| Appearance | yellow crystals hygroscopic |
| Density | 3.7 g/cm3 |
| Melting point | 720 °C (1,328 °F; 993 K) |
| Boiling point | 1,500 °C (2,730 °F; 1,770 K) (decomposes) |
| dissolves | |
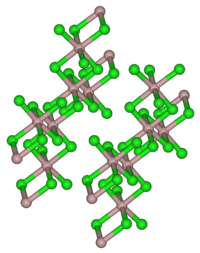
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Related compounds | |
|
Other anions
|
Holmium(III) oxide |
|
Other cations
|
Dysprosium(III) chloride, Erbium(III) chloride |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Holmium(III) chloride is the inorganic compound with the formula HoCl3. It is a common salt but is mainly used in research. It exhibits the same color-changing behavior seen in holmium oxide, being a yellow in natural lighting and a bright pink color in fluorescent lighting.
It forms upon union of the elements, but a more commonly used method involves heating a mixture of holmium(III) oxide and ammonium chloride at 200-250 °C:
In the solid state it has the YCl3 layer structure.
...
Wikipedia
