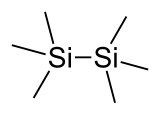
Hexamethyldisilane
 |
|||
|
|
|||
| Identifiers | |||
|---|---|---|---|
|
3D model (JSmol)
|
|||
| 1633463 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.014.465 | ||
| EC Number | 215-911-0 | ||
|
PubChem CID
|
|||
| RTECS number | JM9170000 | ||
| UN number | 1993 | ||
|
|||
|
|||
| Properties | |||
| Si2C6H18 | |||
| Molar mass | 146.39 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 0.715 g/cm3 | ||
| Melting point | 14 °C; 57 °F; 287 K | ||
| Boiling point | 113 °C; 235 °F; 386 K | ||
|
Refractive index (nD)
|
1.422 | ||
| Thermochemistry | |||
|
Std molar
entropy (S |
255.89 J K−1 mol−1 (at 22.52 °C) | ||
| Hazards | |||
| GHS pictograms |
  
|
||
| GHS signal word | DANGER | ||
| H225, H319, H334, H335 | |||
| P210, P261, P305+351+338, P342+311 | |||
| Flash point | 11 °C (52 °F; 284 K) | ||
| Related compounds | |||
|
Related alkylsilanes
|
Tetramethylsilane |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Hexamethyldisilane is the organosilicon compound with the formula Si2(CH3)6, abbreviated Si2Me6. It is a colourless liquid, soluble in organic solvents.
The Si-Si bond in hexamethyldisilane is cleaved by strong nucleophiles and electrophiles. Alkyl lithium compounds react as follows:
Iodine gives trimethylsilyl iodide.
...
Wikipedia


