HMPA
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Hexamethylphosphoramide
|
|
|
Preferred IUPAC name
Hexamethylphosphoric triamide
|
|
| Other names
Hexametapol
HMPA Phosphoric tris(dimethylamide) |
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.010.595 |
| KEGG | |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C6H18N3OP | |
| Molar mass | 179.20 g/mol |
| Appearance | clear, colorless liquid |
| Odor | aromatic, mild, amine-like |
| Density | 1.03 g/cm3 |
| Melting point | 7.20 °C (44.96 °F; 280.35 K) |
| Boiling point | 232.5 °C (450.5 °F; 505.6 K) CRC |
| miscible | |
| Vapor pressure | 0.03 mmHg (20°C) |
| Hazards | |
| Main hazards | carcinogen |
| Safety data sheet | Oxford MSDS |
|
EU classification (DSD)
|
|
| Flash point | 104.4 °C (219.9 °F; 377.5 K) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
none |
|
REL (Recommended)
|
Ca |
|
IDLH (Immediate danger)
|
Ca [N.D.] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
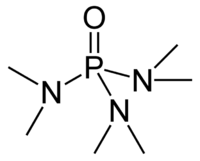
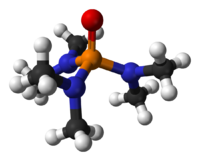
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (i.e. an amide of phosphoric acid) with the formula [(CH3)2N]3PO. This colorless liquid is a useful polar aprotic solvent and additive in organic synthesis.
HMPA is the oxide of the highly basic tertiary phosphine hexamethylphosphorous triamide (HMPT), P(NMe2)3. Like other phosphine oxides (e.g., triphenylphosphine oxide), the molecule has a tetrahedral core and a P-O bond that is highly polarized, with significant negative charge residing on the oxygen atom.
Compounds containing a nitrogen-phosphorus bond typically are degraded by hydrochloric acid to form a protonated amine and phosphate.
HMPA is a specialty solvent for polymers, gases, and organometallic compounds. It improves the selectivity of lithiation reactions by breaking up the oligomers of lithium bases such as butyllithium. Because HMPA selectively solvates cations, it accelerates otherwise slow SN2 reactions by generating more "naked" anions. The basic nitrogen centers in HMPA coordinate strongly to Li+.
HMPA is a ligand in the useful reagents based on molybdenum peroxide complexes, e.g., MoO(O2)2(HMPA)(H2O) is used as an oxidant in organic synthesis.
Dimethyl sulfoxide can often be used in place of HMPA as a solvent. Both are strong hydrogen bond acceptors, and their oxygen atoms bind metal cations. Other alternatives to HMPA include the tetraalkylureas and the cyclic alkylureas like DMPU.
...
Wikipedia
