Glycopyrrolate
 |
|
| Clinical data | |
|---|---|
| Trade names | Robinul (tablets, intravenous), Seebri (inhalation), others |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.008.990 |
| Chemical and physical data | |
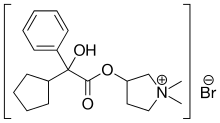
| Formula | C19H28BrNO3 |
| Molar mass | 398.335 g/mol |
| 3D model (Jmol) | |
|
|
|
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602014 |
| Pregnancy category |
|
| Routes of administration |
By mouth, intravenous, inhalation |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 0.6–1.2 hours |
| Excretion | 85% renal, unknown amount in the bile |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.008.990 |
| Chemical and physical data | |
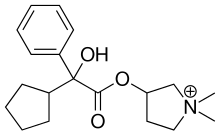
| Formula | C19H28NO3+ |
| Molar mass | 318.431 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Glycopyrronium bromide is a medication of the muscarinic anticholinergic group. It does not cross the blood–brain barrier and consequently has no to few central effects. A synthetic quaternary amine, it is available in oral and intravenous forms and as inhalation. It was developed by Sosei and licensed to Novartis in 2005. The cation, which is the active moiety, is called glycopyrronium (INN) or glycopyrrolate (USAN).
In anesthesia, glycopyrronium injection can be used as a preoperative medication in order to reduce salivary, tracheobronchial, and pharyngeal secretions, as well as decreasing the acidity of gastric secretion. It is also used in conjunction with neostigmine, a neuromuscular blocking reversal agent, to prevent neostigmine's muscarinic effects such as bradycardia.
It is also used to reduce excessive saliva (sialorrhea), and Ménière's disease.
It decreases acid secretion in the stomach and so may be used for treating stomach ulcers, in combination with other medications.
...
Wikipedia
