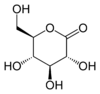
Glucono delta-lactone
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-one
|
|||
| Other names
D-Glucono-1,5-lactone
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.001.833 | ||
| EC Number | 202-016-5 | ||
| E number | E575 (acidity regulators, ...) | ||
| KEGG | |||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C6H10O6 | |||
| Molar mass | 178.14 g·mol−1 | ||
| Melting point | 150–153 °C (302–307 °F; 423–426 K) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Glucono delta-lactone (GDL), also known as gluconolactone, is a food additive with the E number E575 used as a sequestrant, an acidifier, or a curing, pickling, or leavening agent. It is a lactone of D-gluconic acid. Pure GDL is a white odorless crystalline powder.
GDL is commonly found in honey, fruit juices, personal lubricants, wine, and has been marketed for use in feta cheese. GDL is neutral, but hydrolyses in water to gluconic acid which is acidic, adding a tangy taste to foods, though it has roughly a third of the sourness of citric acid. It is metabolized to 6-phospho-D-gluconate; one gram of GDL yields roughly the same amount of metabolic energy as one gram of sugar.
Upon addition to water, GDL is partially hydrolysed to gluconic acid, with the balance between the lactone form and the acid form established as a chemical equilibrium. The rate of hydrolysis of GDL is increased by heat and high pH.
The yeast Saccharomyces bulderi can be used to ferment gluconolactone to ethanol and carbon dioxide. The pH value greatly affects culture growth. Gluconolactone at 1 or 2% in a mineral media solution causes the pH to drop below 3.
...
Wikipedia