Galactose metabolism
 |
|||
|
|
|||
| Identifiers | |||
|---|---|---|---|
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
| MeSH | Galactose | ||
|
PubChem CID
|
|||
| UNII | |||
|
|||
|
|||
| Properties | |||
| C6H12O6 | |||
| Molar mass | 180.156 g mol−1 | ||
| Density | 1.723 g/cm3 | ||
| Melting point | 167 °C (333 °F; 440 K) | ||
| 683.0 g/L | |||
| -103.00·10−6 cm3/mol | |||
| Pharmacology | |||
| V04CE01 (WHO) V08DA02 (WHO) (microparticles) | |||
| Hazards | |||
| NFPA 704 | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
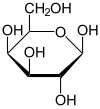
Galactose ( + , "milk sugar"), sometimes abbreviated Gal, is a monosaccharide sugar that is less sweet than glucose and fructose. It is a C-4 epimer of glucose.
Galactan is a polymeric form of galactose found in hemicellulose. Galactan can be converted to galactose by hydrolysis.
Galactose exists in both open-chain and cyclic form. The open-chain form has a carbonyl at the end of the chain.
Four isomers are cyclic, two of them with a pyranose (six-membered) ring, two with a furanose (five-membered) ring. Galactofuranose occurs in bacteria, fungi and protozoa , and is recognized by a putative chordate immune lectin intelectin through its exocyclic 1,2-diol. In the cyclic form there are two anomers, named alpha and beta, since the transition from the open-chain form to the cyclic form involves the creation of a new stereocenter at the site of the open-chain carbonyl. In the beta form, the alcohol group is in the equatorial position, whereas in the alpha form, the alcohol group is in the axial position.
Galactose is a monosaccharide. When combined with glucose (monosaccharide), through a condensation reaction, the result is the disaccharide lactose. The hydrolysis of lactose to glucose and galactose is catalyzed by the enzymes lactase and β-galactosidase. The latter is produced by the lac operon in Escherichia coli.
...
Wikipedia