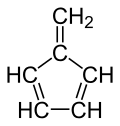
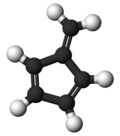
Fulvene
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
5-Methylidenecyclopenta-1,3-diene
|
|||
| Other names
Fulvene
Pentafulvene 5-Methylene-1,3-cyclopentadiene |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C6H6 | |||
| Molar mass | 78.11 g·mol−1 | ||
| -42.9·10−6 cm3/mol | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Fulvene is one of several hydrocarbons with the same formula as benzene, C6H6. The fulvenes are the class of molecules based on this simple hydrocarbon skeleton; the parent chemical, fulvene itself, is rarely encountered.Thiele is credited with discovering the scope of the reaction between cyclopentadiene and aldehydes and ketones that yields the brightly coloured fulvene derivatives. Most fulvenes are still prepared by this route, starting from cyclopentadiene or its sodium cyclopentadienyl anionic form. Modern synthesis of fulvenes now employ protic solvents and catalytic amounts of amines which result in much higher yields.
2,3,4,5-Tetramethylfulvene, abbreviated Me4Fv, is a relatively common ligand in organometallic chemistry. It typically results from the deprotonation of cationic pentamethylcyclopentadienyl complexes. Some Me4Fv complexes are called tuck-in complexes.
...
Wikipedia