Flibanserin
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Addyi |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 33% |
| Protein binding | ~98% |
| Metabolism | Extensive by liver (mainly by CYP3A4 and CYP2C19) |
| Biological half-life | ~11 hours |
| Excretion | Biliary (51%), kidney (44%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.170.970 |
| Chemical and physical data | |
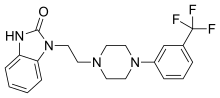
| Formula | C20H21F3N4O |
| Molar mass | 390.40 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Flibanserin, sold under the trade name Addyi, is a medication approved for the treatment of pre-menopausal women with hypoactive sexual desire disorder (HSDD). The medication increases the number of satisfying sexual events per month by about one half over placebo from a starting point of about two to three. The certainty of the estimate is low. The side effects of dizziness, sleepiness, and nausea occur about three to four times more often.
Development by Boehringer Ingelheim was halted in October 2010 following a negative evaluation by the U.S. Food and Drug Administration. The rights to the drug were then transferred to Sprout Pharmaceuticals, which achieved approval of the drug by the US FDA in August 2015.
HSDD was recognized as a distinct sexual function disorder for more than 30 years, but was removed from the Diagnostic and Statistical Manual of Mental Disorders in 2013, and replaced with a new diagnosis called female sexual interest/arousal disorder (FSIAD).
Flibanserin is used for hypoactive sexual desire disorder among women. Those receiving flibanserin report a 0.5 increase compared to placebo in the number of times they had “satisfying sexual events”. In those on flibanserin it rose from 2.8 to 4.5 times a month while women receiving placebo reported also an increase of “satisfying sexual events” from 2.7 to 3.7 times a month. The onset of the flibanserin effect was seen from the first timepoint measured after 4 weeks of treatment and maintained throughout the treatment period.
The effectiveness of flibanserin was evaluated in three phase 3 clinical trials. Each of the three trials had two co-primary endpoints, one for satisfying sexual events (SSEs) and the other for sexual desire. Each of the 3 trials also had a secondary endpoint that measured distress related to sexual desire. All three trials showed that flibanserin produced an increase in the number of SSEs and reduced distress related to sexual desire. The first two trials used an electronic diary to measure sexual desire, and did not find an increase. These two trials also measured sexual desire using the Female Sexual Function index (FSFI) as a secondary endpoint, and an increase was observed using this latter measure. The FSFI was used as the co-primary endpoint for sexual desire in the third trial, and again showed a statistically significant increase.
...
Wikipedia
