Firefly luciferin
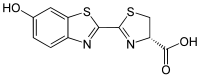 |
|
| Names | |
|---|---|
|
IUPAC name
(4S)-2-(6-hydroxy-1,3-benzothiazol-2-yl)-4,5-dihydrothiazole-4-carboxylic acid
|
|
| Other names
D-(−)-Luciferin
|
|
| Identifiers | |
| 2591-17-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider |
4588411 16735812 One of the other tautomeric representations |
| ECHA InfoCard | 100.018.166 |
| PubChem | 5484207 |
|
|
|
|
| Properties | |
| C11H8N2O3S2 | |
| Molar mass | 280.32 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Firefly luciferin is the luciferin, or light-emitting compound, found in many firefly (Lampyridae) species. It is the substrate of luciferase (EC 1.13.12.7), which is responsible for the characteristic yellow light emission from many firefly species.
As with all other luciferins, oxygen is required to elicit light; however, it has also been found adenosine triphosphate (ATP) and magnesium are required for light emission.
Much of the early work on the chemistry of the firefly luminescence was done in the lab of William D. McElroy at Johns Hopkins University. The luciferin was first isolated and purified in 1949, though it would be several years until a procedure was developed to crystallize the compound in high yield. This, along with the synthesis and structure elucidation, was accomplished by Dr. Emil H. White at the Johns Hopkins University, Department of Chemistry. The procedure was an acid-base extraction, given the carboxylic acid group on the luciferin. The luciferin could be effectively extracted using ethyl acetate at low pH from powder of approximately 15,000 firefly lanterns. The structure was later confirmed by combined use of infrared spectroscopy, UV-vis spectroscopy and synthetic methods to degrade the compound into identifiable fragments.
Crystal luciferin was found to be fluorescent, absorbing ultraviolet light with a peak at 327 nm and emitting light with a peak at 530 nm. Visible emission occurs upon relaxation of the oxyluciferin from a singlet excited state down to its ground state.Alkaline solutions caused a redshift of the absorption likely due to deprotonation of the hydroxyl group on the benzothiazole, but did not affect the fluorescence emission. It was found that the luciferyl adenylate (the AMP ester of luciferin) spontaneously emits light in solution. Different species of fireflies all use the same luciferin, however the color of the light emitted can differ greatly. The light from Photuris pennsylvanica was measured to be 552 nm (green-yellow) while Pyrophorus plagiophthalamus was measured to emit light at 582 nm (orange) in the ventral organ. Such differences are likely due to pH changes or differences in primary structure of the luciferase. Modification of the firefly luciferin substrate has led to "red-shifted" emissions (up to emission wavelength of 675 nm).
...
Wikipedia
