Ferrous oxide
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Iron(II) oxide
|
|
| Other names
Ferrous oxide,iron monoxide
|
|
| Identifiers | |
|
1345-25-1 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:50820 |
| ChemSpider |
14237 |
| ECHA InfoCard | 100.014.292 |
| 13590 | |
| PubChem | 14945 |
| UNII |
G7036X8B5H |
|
|
|
|
| Properties | |
| FeO | |
| Molar mass | 71.844 g/mol |
| Appearance | black crystals |
| Density | 5.745 g/cm3 |
| Melting point | 1,377 °C (2,511 °F; 1,650 K) |
| Boiling point | 3,414 °C (6,177 °F; 3,687 K) |
| Insoluble | |
| Solubility | insoluble in alkali, alcohol dissolves in acid |
| +7200·10−6 cm3/mol | |
|
Refractive index (nD)
|
2.23 |
| Hazards | |
| Main hazards | can be pyrophoric |
| Safety data sheet | ICSC 0793 |
| NFPA 704 | |
| variable | |
| Related compounds | |
|
Other anions
|
iron(II) fluoride, iron(II) sulfide, iron(II) selenide, iron(II) telluride |
|
Other cations
|
manganese(II) oxide, cobalt(II) oxide |
|
Related compounds
|
Iron(III) oxide, Iron(II,III) oxide |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Iron(II) oxide or ferrous oxide is the inorganic compound with the formula FeO. Its mineral form is known as wüstite. One of several iron oxides, it is a black-colored powder that is sometimes confused with rust, which consists of hydrated iron(III) oxide (ferric oxide). Iron(II) oxide also refers to a family of related non-stoichiometric compounds, which are typically iron deficient with compositions ranging from Fe0.84O to Fe0.95O.
FeO can be prepared by the thermal decomposition of iron(II) oxalate.
The procedure is conducted under an inert atmosphere to avoid the formation of ferric oxide. A similar procedure can also be used for the synthesis of manganous oxide and stannous oxide.
Stoichiometric FeO can be prepared by heating Fe0.95O with metallic iron at 770 °C and 36 kbar.
FeO is thermodynamically unstable below 575 °C, tending to disproportionate to metal and Fe3O4:
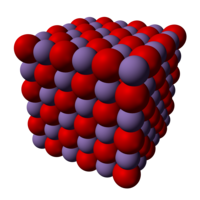
Iron(II) oxide adopts the cubic, rock salt structure, where iron atoms are octahedrally coordinated by oxygen atoms and the oxygen atoms octahedrally coordinated by iron atoms. The non-stoichiometry occurs because of the ease of oxidation of FeII to FeIII effectively replacing a small portion of FeII with two thirds their number of FeIII, which take up tetrahedral positions in the close packed oxide lattice.
Below 200 K there is a minor change to the structure which changes the symmetry to rhombohedral and samples become antiferromagnetic.
Iron(II) oxide makes up approximately 9% of the Earth's mantle. Within the mantle, it may be electrically conductive, which is a possible explanation for perturbations in Earth's rotation not accounted for by accepted models of the mantle's properties.
...
Wikipedia

