Iron(II) oxalate
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
Iron(II) oxalate
|
|
| Other names
Iron oxalate
Ferrous oxalate |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.007.472 |
| EC Number | 208-217-4 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| FeC2O4 (anhydrous) FeC2O4·2 H2O (dihydrate) |
|
| Molar mass | 143.86 g/mol (anhydrous) 179.89 g/mol (dihydrate) |
| Appearance | yellow powder |
| Odor | odorless |
| Density | 2.28 g/cm3 |
| Melting point | 190 °C (374 °F; 463 K) (anhydrous) 150–160 °C (302–320 °F; 423–433 K) (dihydrate) decomposes |
| Boiling point | 365.1 °C (689.2 °F; 638.2 K) (anhydrous) |
| dihydrate: 0.097 g/100ml (25 °C) |
|
| Hazards | |
| GHS pictograms |  |
| GHS signal word | Warning |
| H302, H312 | |
| P280 | |
| Flash point | 188.8 °C (371.8 °F; 461.9 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Ferrous oxalate, or iron(II) oxalate, is a inorganic compound with the formula FeC2O4(H2O)x where x is typically 2. These are orange compounds, poorly soluble in water.
The dihydrate FeC2O4(H2O)2 is a coordination polymer, consisting of chains of oxalate-bridged ferrous centers, each with two aquo ligands.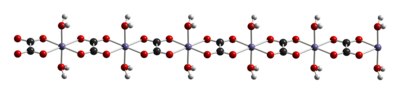
When heated, it dehydrates and decomposes into a mixture of iron oxides and pyrophoric iron metal, with release of carbon dioxide, carbon monoxide, and water.
A number of other iron oxalates are known
...
Wikipedia
