Ferrodoxin
| 2Fe-2S iron-sulfur cluster binding domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
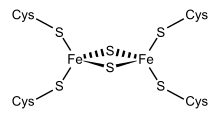
Structural representation of an Fe2S2 ferredoxin.
|
|||||||||
| Identifiers | |||||||||
| Symbol | Fer2 | ||||||||
| Pfam | PF00111 | ||||||||
| InterPro | IPR001041 | ||||||||
| PROSITE | PDOC00642 | ||||||||
| SCOP | 3fxc | ||||||||
| SUPERFAMILY | 3fxc | ||||||||
| OPM protein | 1kf6 | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
| ferredoxin 1 | |
|---|---|
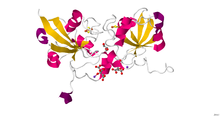
Crystal structure of human ferredoxin-1 (FDX1).
|
|
| Identifiers | |
| Symbol | FDX1 |
| Alt. symbols | FDX |
| Entrez | 2230 |
| HUGO | 3638 |
| OMIM | 103260 |
| RefSeq | NM_004109 |
| UniProt | P10109 |
| Other data | |
| Locus | Chr. 11 q22.3 |
| 3Fe-4S binding domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|

Structural representation of an Fe3S4 ferredoxin.
|
|||||||||
| Identifiers | |||||||||
| Symbol | Fer4 | ||||||||
| Pfam | PF00037 | ||||||||
| InterPro | IPR001450 | ||||||||
| PROSITE | PDOC00176 | ||||||||
| SCOP | 5fd1 | ||||||||
| SUPERFAMILY | 5fd1 | ||||||||
| OPM protein | 1kqf | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
Ferredoxins (from Latin ferrum: iron + redox, often abbreviated "fd") are iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium Clostridium pasteurianum.
Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight-excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase EC 1.18.1.2.
Ferredoxins are small proteins containing iron and sulfur atoms organized as iron-sulfur clusters. These biological "capacitors" can accept or discharge electrons, with the effect of a change in the oxidation state of the iron atoms between +2 and +3. In this way, ferredoxin acts as an electron transfer agent in biological redox reactions.
Other bioinorganic electron transport systems include rubredoxins, , blue copper proteins, and the structurally related Rieske proteins.
...
Wikipedia
