Etretinate
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Tigason, formerly Tegison |
| AHFS/Drugs.com | Drugs.com archive |
| MedlinePlus | a601010 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | >99% |
| Metabolites | Free acid, Z-form, chain shortening |
| Biological half-life | 120 days |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.053.727 |
| Chemical and physical data | |
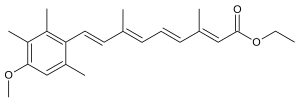
| Formula | C23H30O3 |
| Molar mass | 354.483 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Etretinate (trade name Tegison) is a medication developed by Hoffmann–La Roche that was approved by the FDA in 1986 to treat severe psoriasis. It is a second-generation retinoid. It was subsequently removed from the Canadian market in 1996 and the United States market in 1998 due to the high risk of birth defects. It remains on the market in Japan as Tigason.
Etretinate is a highly lipophilic, aromatic retinoid. It is stored and released from adipose tissue, so its effects can continue long after dosage stops. It is detectable in the plasma for up to three years following therapy. Etretinate has a low therapeutic index and a long elimination half-life (t1/2) of 120 days, which make dosing difficult.
Etretinate has been replaced by acitretin, the free acid (without the ethyl ester). While acitretin is less lipophilic and has a half-life of only 50 hours, it is partly metabolized to etretinate in the body, so that it is still a long-acting teratogen and pregnancy is prohibited for two years after therapy.
Side effects are those typical of hypervitaminosis A, most commonly
The drug was approved by the FDA in 1986 to treat severe psoriasis. It was subsequently removed from the Canadian market in 1996 and the United States market in 1998 due to the high risk of birth defects.
In Japan, the drug remains on market branded Tigason.
...
Wikipedia
