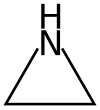
Ethyleneimine
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Aziridine
|
|||
| Other names
Azacyclopropane, Ethylene imine, Aminoethylene, Azirane, Dimethyleneimine, Dimethylenimine, Ethylimine
|
|||
| Identifiers | |||
|
151-56-4 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:30969 |
||
| ChEMBL |
ChEMBL540990 |
||
| ChemSpider |
8682 |
||
| ECHA InfoCard | 100.005.268 | ||
| EC Number | 205-793-9 | ||
| KEGG |
C11687 |
||
|
|||
|
|||
| Properties | |||
| C2H5N | |||
| Molar mass | 43.07 g·mol−1 | ||
| Appearance | Clear colorless oily liquid | ||
| Odor | ammonia-like | ||
| Density | 0.8321 g/mL 20 °C | ||
| Melting point | −77.9 °C (−108.2 °F; 195.2 K) | ||
| Boiling point | 56 °C (133 °F; 329 K) | ||
| miscible | |||
| Vapor pressure | 160 mmHg (20° C) | ||
| Hazards | |||
| Main hazards | highly flammable, very toxic | ||
| NFPA 704 | |||
| Flash point | −11 °C (12 °F; 262 K) | ||
| 322 °C (612 °F; 595 K) | |||
| Explosive limits | 3.6–46% | ||
| Lethal dose or concentration (LD, LC): | |||
|
LC50 (median concentration)
|
250 ppm (rat, 1 hr) 250 ppm (guinea pig, 1 hr) 62 ppm (rat, 4 hr) 223 ppm (mouse, 2 hr) 56 ppm (rat, 2 hr) 2236 ppm (mouse, 10 min) |
||
|
LCLo (lowest published)
|
25 ppm (guinea pig, 8 hr) 56 ppm (rabbit, 2 hr) |
||
| US health exposure limits (NIOSH): | |||
|
PEL (Permissible)
|
OSHA-Regulated Carcinogen | ||
|
REL (Recommended)
|
Ca | ||
|
IDLH (Immediate danger)
|
Ca [100 ppm] | ||
| Related compounds | |||
|
Related heterocycles
|
Borirane Ethylene oxide Thiirane |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Aziridines are organic compounds containing the aziridine functional group, a three-membered heterocycle with one amine group (-NH-) and two methylene bridges (-CH
2-). The parent compound is aziridine (or ethylene imine), with molecular formula C
2H
5N.
The bond angles in aziridine are approximately 60°, considerably less than the normal hydrocarbon bond angle of 109.5°, which results in angle strain as in the comparable cyclopropane and ethylene oxide molecules. A banana bond model explains bonding in such compounds. Aziridine is less basic than acyclic aliphatic amines, with a pKa of 7.9 for the conjugate acid, due to increased s character of the nitrogen free electron pair. Angle strain in aziridine also increases the barrier to nitrogen inversion. This barrier height permits the isolation of separate invertomers, for example the cis and trans invertomers of N-chloro-2-methylaziridine.
...
Wikipedia