Emtricitabine
 |
|
| Clinical data | |
|---|---|
| Trade names | Emtriva |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604004 |
| Pregnancy category |
|
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 93% |
| Protein binding | Very low (less than 4%) |
| Metabolism |
Hepatic oxidation and glucuronidation system not involved |
| Biological half-life | 10 hours |
| Excretion | Renal (86%) and fecal (14%) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| ECHA InfoCard | 100.120.945 |
| Chemical and physical data | |
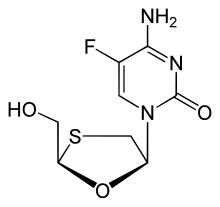
| Formula | C8H10FN3O3S |
| Molar mass | 247.248 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Emtricitabine (2'-deoxy-5-fluoro-3'thiacytidine,FTC), with trade name Emtriva (formerly Coviracil), is a nucleoside reverse transcriptase inhibitor (NRTI) for the treatment of HIV infection in adults and children.
Emtricitabine is also marketed in a fixed-dose combination with tenofovir (Viread) under the brand name Truvada.
A fixed-dose triple combination of emtricitabine, tenofovir and efavirenz (Sustiva, marketed by Bristol-Myers Squibb) was approved by the U.S. Food and Drug Administration (FDA) on July 12, 2006 under the brand name Atripla.
Emtricitabine makes up one fourth of the Quad pill (brand name: Stribild).
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.
Emtricitabine is indicated in combination with other antiretroviral agents for the treatment of HIV infection in adults. Emtricitabine is commercially available and is approved by the FDA for treatment of HIV infection.
Emtricitabine exhibits clinical activity against the hepatitis B virus (HBV), but is not approved by the FDA for the treatment of HBV infection. Among individuals with chronic HBV infection, emtricitabine treatment results in significant histologic, virologic, and biochemical improvement. The safety profile of emtricitabine during treatment is similar to that of a placebo. Emtricitabine, like all other FDA approved drugs, cures neither HIV nor HBV infection. In a study involving individuals with HBV infection, symptoms of infection returned in 23% of emtricitabine-treated individuals who were taken off therapy. In studies involving individuals with chronic HIV infection, viral replication also resumes when study subjects are taken off therapy. As with drugs used to treat HIV infection, drugs used to treat HBV infection may have to be used in combination to prevent the evolution of drug resistant strains. The effectiveness of emtricitabine in combination with other anti-HBV drugs has not been established.
...
Wikipedia
