Electrons
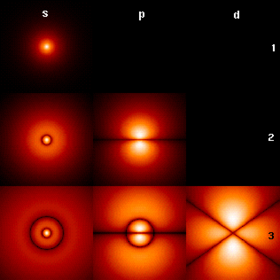
Hydrogen atom orbitals at different energy levels. The brighter areas are where one is most likely to find an electron at any given time.
|
|
| Composition | Elementary particle |
|---|---|
| Statistics | Fermionic |
| Generation | First |
| Interactions | Gravity, electromagnetic, weak |
| Symbol |
e− , β− |
| Antiparticle | Positron (also called antielectron) |
| Theorized |
Richard Laming (1838–1851), G. Johnstone Stoney (1874) and others. |
| Discovered | J. J. Thomson (1897) |
| Mass |
9.10938356(11)×10−31 kg 5.48579909070(16)×10−4 u [1822.8884845(14)]−1 u 0.5109989461(31) MeV/c2 |
| Mean lifetime | stable ( > 6.6×1028 yr) |
| Electric charge |
−1 e −1.6021766208(98)×10−19 C −4.80320451(10)×10−10 esu |
| Magnetic moment | −1.00115965218091(26) μB |
| Spin | 1/2 |
| Weak isospin | LH: −1/2, RH: 0 |
| Weak hypercharge | LH: -1, RH: −2 |
The electron is a subatomic particle, symbol
e−
or
β−
, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton.Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. As it is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a larger De Broglie wavelength for a given energy.
...
Wikipedia
