Ebastine
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | Greater than 95% |
| Metabolism | Hepatic (CYP3A4-mediated), to carebastine |
| Biological half-life | 15 to 19 hours (carebastine) |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.106.831 |
| Chemical and physical data | |
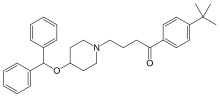
| Formula | C32H39NO2 |
| Molar mass | 469.658 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Ebastine (trade names Evastin, Kestine, Ebastel, Aleva, Ebatrol) is a H1antihistamine with low potential for causing drowsiness.
It does not penetrate the blood–brain barrier to a significant amount and thus combines an effective block of the H1 receptor in peripheral tissue with a low incidence of central side effects, i.e. seldom causing sedation or drowsiness.
The patent in which the structure of ebastine is first mentioned is EP 134124 in Europe and US 4550116 in the US. The substance is often provided in micronised form due to poor water solubility.
Ebastine is a second-generation H1 receptor antagonist that is indicated mainly for allergic rhinitis and chronic idiopathic urticaria. It is available in 10 and 20 mg tablets and as fast-dissolving tablets, as well as in pediatric syrup. It has a recommended flexible daily dose of 10 or 20 mg, depending on disease severity.
Ebastine is available in different formulations (tablets, fast dissolving tablets and syrup) and commercialized under different brand names around the world,Ebatrol, Ebet, Ebastel FLAS, Kestine, KestineLIO, KestinLYO, EstivanLYO, Evastel Z, etc.
After oral administration, ebastine undergoes extensive first-pass metabolism by hepatic into its active carboxylic acid metabolite, carebastine. This conversion is practically complete.
Data from over 8,000 patients in more than 40 clinical trials and studies suggest efficacy of ebastine in the treatment of intermittent allergic rhinitis, persistent allergic rhinitis and other indications.
...
Wikipedia
