EDDS
 |
|
| Names | |
|---|---|
|
IUPAC name
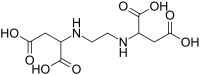
Ethylenediamine-N,N′-disuccinic acid
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | N,N'-ethylenediamine+disuccinic+acid |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C10H16N2O8 | |
| Molar mass | 292.24 g·mol−1 |
| Density | 1.44 g mL−1 |
| Melting point | 220 to 222 °C (428 to 432 °F; 493 to 495 K) |
| Acidity (pKa) | 2.4 |
| Basicity (pKb) | 11.6 |
| Thermochemistry | |
|
Std enthalpy of
formation (ΔfH |
−1.9541–−1.9463 MJ mol−1 |
|
Std enthalpy of
combustion (ΔcH |
−4.2755–−4.2677 MJ mol−1 |
| Related compounds | |
|
Related alkanoic acids
|
EDTA |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Ethylenediamine-N,N'-disuccinic acid (EDDS) is an aminopolycarboxylic acid. It is a colourless solid that is used as chelating agent that may offer a biodegradable alternative to EDTA, which is currently used on a large scale in numerous applications.
EDDS has two chiral centers, and as such three stereoisomers. These are the enantiomeric (R,R) and (S,S) isomers and the achiral meso (R,S) isomer. As a biodegradable replacement for EDTA, only the (S,S) stereoisomer is of interest. The (R,S) and (R,R) stereoisomers are less biodegradable, whereas the (S,S) stereoisomer has been shown to be very effectively biodegraded even in highly polluted soils.
EDDS was first synthesized from maleic acid and ethylenediamine. Some microorganisms have been manipulated for industrial-scale synthesis of (S,S)-EDDS from ethylenediamine and fumaric acid or maleic acid, which proceeds as follows:
(S,S)-EDDS is produced stereospecifically by the alkylation of an ethylenedibromide with L-aspartic acid. Racemic EDDS is produced by the reaction of ethylenediamine with fumaric acid or maleic acid.
In comparing the effectiveness of (S,S)-EDDS versus EDTA as chelating agents for iron(III):
Because of the lower stability for [Fe(S,S)-EDDS]−, the useful range being roughly 3<pH(S,S)-EDDS<9 and 2<pHEDTA<11. However, this range is sufficient for most applications.
Another comparison that can be made between (S,S)-EDDS and EDTA is the structure of the chelated complex. EDTA’s six donor sites form five five-membered chelate rings around the metal ion, four NC2OFe rings and one C2N2Fe ring. The C2N2Fe ring and two of NC2OFe rings define a plane, and two NC2OFe rings are perpendicular to the plane that contains the C2-symmetry axis. The five-membered rings are slightly strained. EDDS’s six donor sites form both five- and six-membered chelate rings around the metal ion: two NC2OFe rings, two NC3OFe rings, and one C2N2Fe ring. Studies of the crystal structure of the Fe[(S,S)-EDDS]− complex show that the two five-membered NC3OFe rings project out of the plane of the complex, reducing the equatorial ring strain that exists in the Fe[EDTA]− complex. The complex also has C2 symmetry.
...
Wikipedia
