Dobutamine
 |
|
| Clinical data | |
|---|---|
| Trade names | Dobutrex |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682861 |
| Pregnancy category |
|
| Routes of administration |
Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Biological half-life | 2 minutes |
| Identifiers | |
|
|
| Synonyms | Dobutrex, Inotrex |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
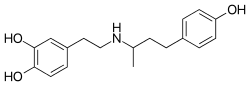
| Formula | C18H23NO3 |
| Molar mass | 301.38 g/mol |
| 3D model (Jmol) | |
| Chirality | Racemic mixture |
|
|
|
|
Dobutamine is a sympathomimetic drug used in the treatment of heart failure and cardiogenic shock. Its primary mechanism is direct stimulation of β1 receptors of the sympathetic nervous system. Dobutamine was developed in the 1970s by Drs. Ronald Tuttle and Jack Mills at Eli Lilly and Company, as a structural analogue of isoprenaline.
Dobutamine is used to treat acute but potentially reversible heart failure, such as which occurs during cardiac surgery or in cases of septic or cardiogenic shock, on the basis of its positive inotropic action.
Dobutamine can be used in cases of congestive heart failure to increase cardiac output. It is indicated when parenteral therapy is necessary for inotropic support in the short-term treatment of patients with cardiac decompensation due to depressed contractility, which could be the result of either organic heart disease or cardiac surgical procedures. It is not useful in ischemic heart disease because it increases heart rate and thus increases myocardial oxygen demand.
The drug is also commonly used in the hospital setting as a pharmacologic stress testing agent to identify coronary artery disease.
Primary side effects include those commonly seen for β1 active sympathomimetics, such as hypertension, angina, arrhythmia, and tachycardia. Used with caution in atrial fibrillation as it has the effect of increasing the atrioventricular (AV) conduction.
...
Wikipedia
