Diphosphorus pentoxide
|
|
|||
 |
|||
| Names | |||
|---|---|---|---|
| Other names
Diphosphorus pentoxide
Phosphorus(V) oxide Phosphoric anhydride Tetraphosphorus decaoxide Tetraphosphorus decoxide |
|||
| Identifiers | |||
|
1314-56-3 16752-60-6 (P4O10) |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:37376 |
||
| ChemSpider |
14128 |
||
| ECHA InfoCard | 100.013.852 | ||
| PubChem | 14812 | ||
| RTECS number | TH3945000 | ||
|
|||
|
|||
| Properties | |||
| P4O10 | |||
| Molar mass | 283.886 g mol−1 | ||
| Appearance | white powder very deliquescent pungent odour |
||
| Density | 2.39 g/cm3 | ||
| Melting point | sublimes | ||
| Boiling point | 360 °C (680 °F; 633 K) | ||
| exothermic hydrolysis | |||
| Vapor pressure | 1 mmHg @ 385 °C (stable form) | ||
| Hazards | |||
| Safety data sheet | MSDS | ||
|
EU classification (DSD)
|
not listed | ||
| NFPA 704 | |||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Phosphorus pentoxide is a chemical compound with molecular formula P4O10 (with its common name derived from its empirical formula, P2O5). This white crystalline solid is the anhydride of phosphoric acid. It is a powerful desiccant and dehydrating agent.
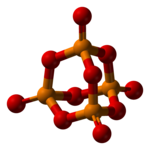
Phosphorus pentoxide crystallizes in at least four forms or polymorphs. The most familiar one, a metastable form, shown in the figure, comprises molecules of P4O10. Weak van der Waals forces hold these molecules together in a hexagonal lattice (However, in spite of the high symmetry of the molecules, the crystal packing is not a close packing). The structure of the P4O10 cage is reminiscent of adamantane with Tdsymmetry point group. It is closely related to the corresponding anhydride of phosphorous acid, P4O6. The latter lacks terminal oxo groups. Its density is 2.30 g/cm3. It boils at 423 °C under atmospheric pressure; if heated more rapidly it can sublimate. This form can be made by condensing the vapor of phosphorus pentoxide rapidly, the result is an extremely hygroscopic solid.
The other polymorphs are polymeric, but in each case the phosphorus atoms are bound by a tetrahedron of oxygen atoms, one of which forms a terminal P=O bond involving the donation of the terminal oxygen p-orbital electrons to the antibonding phosphorus-oxygen single bonds. The macromolecular form can be made by heating the compound in a sealed tube for several hours, and maintaining the melt at a high temperature before cooling the melt to the solid. The metastable orthorhombic, "O"-form (density 2.72 g/cm3, melting point 562 °C), adopts a layered structure consisting of interconnected P6O6 rings, not unlike the structure adopted by certain polysilicates. The stable form is a higher density phase, also orthorhombic, the so-called O' form. It consists of a 3-dimensional framework, density 3.5 g/cm3. The remaining polymorph is a glass or amorphous form; it can be made by fusing any of the others.
...
Wikipedia