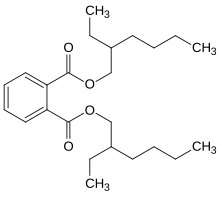
Dioctyl phthalate
 |
|
| Names | |
|---|---|
|
Preferred IUPAC name
Bis(2-ethylhexyl) benzene-1,2-dicarboxylate
|
|
| Other names
Bis(2-ethylhexyl) phthalate
Di-sec octyl phthalate DEHP Octyl phthalate |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.829 |
| KEGG | |
| UNII | |
|
|
|
|
| Properties | |
| C24H38O4 | |
| Molar mass | 390.56 g·mol−1 |
| Appearance | colorless, oily liquid |
| Density | 0.99 g/mL (20°C) |
| Melting point | −50 °C (−58 °F; 223 K) |
| Boiling point | 385 °C (725 °F; 658 K) |
| 0.00003% (23.8°C) | |
| Vapor pressure | <0.01 mmHg (20°C) |
| Hazards | |
| Main hazards | Carcinogen, irritant, teratogen |
| NFPA 704 | |
| Flash point | 216 °C; 420 °F; 489 K (open cup) |
| Explosive limits | 0.3%-? |
| Lethal dose or concentration (LD, LC): | |
|
LD50 (median dose)
|
34,000 mg/kg (oral, rabbit) 26,000 mg/kg (oral, guinea pig) 30,600 mg/kg (oral, rat) 30,000 mg/kg (oral, mouse) |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
TWA 5 mg/m3 |
|
REL (Recommended)
|
Ca TWA 5 mg/m3 ST 10 mg/m3 |
|
IDLH (Immediate danger)
|
Ca [5000 mg/m3] |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Bis(2-ethylhexyl) phthalate (di-2-ethylhexyl phthalate, diethylhexyl phthalate, DEHP; dioctyl phthalate, DOP) is an organic compound with the formula C6H4(CO2C8H17)2. DEHP is the most common member of the class of phthalates, which are used as plasticizers. It is the diester of phthalic acid and the branched-chain 2-ethylhexanol. This colorless viscous liquid is soluble in oil, but not in water. Accounting for an almost 54% market share in 2010, DEHP is a high production volume chemical.
Due to its suitable properties and the low cost, DEHP is widely used as a plasticizer in manufacturing of articles made of PVC. Plastics may contain 1% to 40% of DEHP. It is also used as a hydraulic fluid and as a dielectric fluid in capacitors. DEHP also finds use as a solvent in glowsticks.
Approximately three billion kilograms are produced annually worldwide. It is estimated that at least 241 million pounds of DEHP were produced in the US in 1999.
Industrial production entails the reaction of phthalic anhydride with 2-ethylhexanol:
2-Ethylhexanol is chiral, and the resultant DEHP consists of a mixture of (R,R)-, (S,S)-, and (R,S)-isomers (left).
Manufacturers of flexible PVC articles can choose among several alternative plasticizers offering similar technical properties as DEHP. These alternatives include other phthalates such as diisononyl phthalate (DINP), di-2-propyl heptyl phthalate (DPHP), diisodecyl phthalate (DIDP), and non-phthalates such as 1,2-cyclohexane dicarboxylic acid diisononyl ester (DINCH), dioctyl terephthalate (DOTP), and citrate esters.
...
Wikipedia

