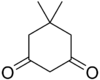
Dimedone
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
5,5-Dimethylcyclohexane-1,3-dione
|
|||
| Other names
Cyclomethone,
5,5-dimethyl-1,3-cyclohexanedione, Dimethyldihydroresorcinol, Methone |
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.004.369 | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| C8H12O2 | |||
| Molar mass | 140.17968 | ||
| Appearance | Yellow crystals | ||
| Melting point | 147 to 150 °C (297 to 302 °F; 420 to 423 K) (decomposes) | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Dimedone is a cyclic diketone used in organic chemistry to determine whether a compound contains an aldehyde group. Cyclohexanediones in general can be used as catalysts in the formation of transition-metal complexes. Other uses include applications in colorimetry, crystallography, luminescence and spectrophotometric analysis. It can also be used for chemistry involving organic compounds of low electrical resistance.
Dimedone is prepared from mesityl oxide and dimethyl malonate.
Dimedone usually comes in the form of white crystals. It is stable under ambient conditions and soluble in water, as well as ethanol and methanol. It has a melting point range of 147–150 °C (420–423 K).
Dimedone is in equilibrium with its tautomer in solution — in a 2:1 keto to enol ratio in chloroform.
Crystalline dimedone contains chains of molecules, in the enol form, linked by hydrogen bonds:
...
Wikipedia