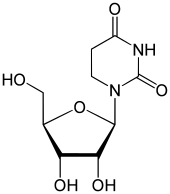
Dihydrouridine
 |
|
| Names | |
|---|---|
|
IUPAC name
1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-diazinane-2,4-dione
|
|
| Other names
3,4-dihydrouridine
3,4,5,6-tetrahydrouridine 5,6-dihydrouridine |
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C9H14N2O6 | |
| Molar mass | 246.217 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Dihydrouridine (abbreviated as D, DHU, or UH2) is a pyrimidine which is the result of adding two hydrogen atoms to a uridine, making it a fully saturated pyrimidine ring with no remaining double bonds. D is found in tRNA and rRNA molecules as a nucleoside; the corresponding nucleobase is 5,6-dihydrouracil.
Because it is non-planar, D disturbs the stacking interactions in helices and destabilizes the RNA structure. D also stabilizes the C2’-endo sugar conformation, which is more flexible than the C3’-endo conformation, and this effect is propagated to the 5’-neighboring residue. Thus, while pseudouridine and 2’-O-methylations stabilize the local RNA structure, D does the opposite.
tRNA of organisms that grow at low temperatures (psychrophiles) have high 5,6-dihydrouridine levels (40-70% more on average) which provides the necessary, local, flexibility of the tRNA at or below the freezing point.
...
Wikipedia
