Dihydroartemisinin
 |
|
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration |
Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Liver |
| Biological half-life | About 4-11 hours |
| Excretion | Mainly Bile |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.128.242 |
| Chemical and physical data | |
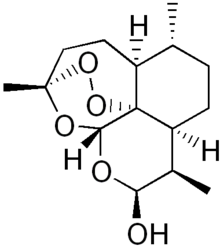
| Formula | C15H24O5 |
| Molar mass | 284.35 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Dihydroartemisinin (also known as dihydroqinghaosu, artenimol or DHA) is a drug used to treat malaria. Dihydroartemisinin is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also available as a drug in itself. It is a semi-synthetic derivative of artemisinin and is widely used as an intermediate in the preparation of other artemisinin-derived antimalarial drugs. It is sold commercially in combination with piperaquine and has been shown to be equivalent to artemether/lumefantrine.
Dihydroartemisinin is used to treat malaria, generally as a combination drug with piperaquine.
In a systematic review of randomized controlled trials, both dihydroartemisinin-piperaquine and artemether-lumefantrine are very effective at treating malaria (high quality evidence). However, dihydroartemisinin-piperaquine cures slightly more patients than artemether-lumefantrine, and it also prevents further malaria infections for longer after treatment (high quality evidence). Dihydroartemisinin-piperaquine and artemether-lumefantrine probably have similar side effects (moderate quality evidence). The studies were all conducted in Africa. In studies of people living in Asia, dihydroartemisinin-piperaquine is as effective as artesunate plus mefloquine at treating malaria (moderate quality evidence). Artesunate plus mefloquine probably causes more nausea, vomiting, dizziness, sleeplessness, and palpitations than dihydroartemisinin-piperaquine (moderate quality evidence).
The proposed mechanism of action of artemisinin involves cleavage of endoperoxide bridges by iron, producing free radicals (hypervalent iron-oxo species, epoxides, aldehydes, and dicarbonyl compounds) which damage biological macromolecules causing oxidative stress in the cells of the parasite. Malaria is caused by apicomplexans, primarily Plasmodium falciparum, which largely reside in red blood cells and itself contains iron-rich heme-groups (in the form of hemozoin). In 2015 artemisinin was shown to bind to a large number targets suggesting that it acts in a promiscuous manner.Recent mechanism research discovered that artemisinin targets a broad spectrum of proteins in the human cancer cell proteome through heme-activated radical alkylation.
...
Wikipedia
