Artemisinin
 |
|
 |
|
| Clinical data | |
|---|---|
| Pronunciation | /ɑːrtᵻˈmɪsᵻnᵻn/ |
| Routes of administration |
Oral |
| ATC code | |
| Identifiers | |
|
|
| Synonyms | Artemisinine, qinghaosu |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.110.458 |
| Chemical and physical data | |
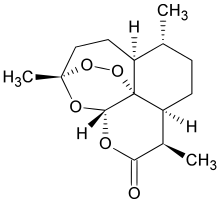
| Formula | C15H22O5 |
| Molar mass | 282.332 g/mol |
| 3D model (Jmol) | |
| Density | 1.24 ± 0.1 g/cm3 |
| Melting point | 152 to 157 °C (306 to 315 °F) |
| Boiling point | decomposes |
|
|
|
|
|
|
|
Artemisinin, also known as qinghao su (Chinese: 青蒿素), and its semi-synthetic derivatives are a group of drugs used against Plasmodium falciparum malaria. It was discovered by Tu Youyou, a Chinese scientist, who was awarded half of the 2015 Nobel Prize in Medicine for her discovery. Treatments containing an artemisinin derivative (artemisinin-combination therapies, ACTs) are now standard treatment worldwide for P. falciparum malaria. Artemisinin is isolated from the plant Artemisia annua, sweet wormwood, an herb employed in Chinese traditional medicine. A precursor compound can be produced using genetically engineered yeast.
Chemically, artemisinin is a sesquiterpene lactone containing an unusual peroxide bridge. This peroxide is believed to be responsible for the drug's mechanism of action. Few other natural compounds with such a peroxide bridge are known.
Artemisinin and its endoperoxides derivatives have been used for the treatment of P. falciparum related infections but low bioavailability, poor pharmacokinetic properties and high cost of the drugs are a major drawback of their use. Use of the drug by itself as a monotherapy is explicitly discouraged by the World Health Organization, as there have been signs that malarial parasites are developing resistance to the drug. Therapies that combine artemisinin or its derivatives with some other antimalarial drug are the preferred treatment for malaria and are both effective and well tolerated in patients.
...
Wikipedia
