Diazoxide
 |
|
| Clinical data | |
|---|---|
| Trade names | Proglycem |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration |
Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 90% |
| Metabolism | Hepatic oxidation and sulfate conjugation |
| Biological half-life | 21-45 hours |
| Excretion | Renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.006.063 |
| Chemical and physical data | |
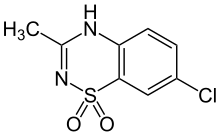
| Formula | C8H7ClN2O2S |
| Molar mass | 230.672 g/mol |
| 3D model (Jmol) | |
|
|
|
|
Diazoxide (INN; brand name Proglycem) is a potassium channel activator, which causes local relaxation in smooth muscle by increasing membrane permeability to potassium ions. This switches off voltage-gated calcium ion channels, preventing calcium flux across the sarcolemma and activation of the contractile apparatus.
In the United States, this agent is typically given in hospital.
Diazoxide is used as a vasodilator in the treatment of acute hypertension or malignant hypertension.
Diazoxide also inhibits the secretion of insulin from the pancreas, thus it is used to counter hypoglycemia in disease states such as insulinoma (a tumor producing insulin) or congenital hyperinsulinism.
Diazoxide acts as a positive allosteric modulator of the AMPA and kainate receptors, suggesting potential application as a cognitive enhancer.
Diazoxide interferes with insulin release through its action on potassium channels. Diazoxide is one of the most potent openers of the K+ ATP channels present on the insulin producing beta cells of the pancreas. Opening these channels leads to hyperpolarization of cell membrane, a decrease in calcium influx, and a subsequently reduced release of insulin. This mechanism of action is the mirror opposite of that of Sulfonylureas, a class of medications used to increase insulin release in Type 2 Diabetics. Therefore, this medicine is not given to non-insulin dependent diabetic patients.
The Food and Drug Administration published a Safety Announcement in July 2015 highlighting the potential for development of pulmonary hypertension in newborns and infants treated with this drug.
...
Wikipedia
