Diazinon
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
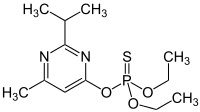
O,O-Diethyl O-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate
|
|
| Other names
Diethoxy-[(2-isopropyl-6-methyl-4-pyrimidinyl)oxy]-thioxophosphorane
Basudin Diazide Spectracide |
|
| Identifiers | |
|
333-41-5 |
|
| 3D model (Jmol) | Interactive image |
| ChEBI |
CHEBI:34682 |
| ChEMBL |
ChEMBL388560 |
| ChemSpider |
2909 |
| ECHA InfoCard | 100.005.795 |
| KEGG |
D07856 |
| PubChem | 3017 |
| UNII |
YUS1M1Q929 |
|
|
|
|
| Properties | |
| C12H21N2O3PS | |
| Molar mass | 304.34 g·mol−1 |
| Appearance | Colorless to dark brown liquid |
| Odor | faint, ester-like |
| Density | 1.116-1.118 g/cm3 at 20 °C |
| Boiling point | decomposes |
| 40 mg/L | |
| log P | 3.81 (octanol/water) |
| Pharmacology | |
| QP53AF03 (WHO) | |
| Hazards | |
| Flash point | 82 °C; 180 °F; 355 K |
| US health exposure limits (NIOSH): | |
|
PEL (Permissible)
|
none |
|
REL (Recommended)
|
TWA 0.1 mg/m3 [skin] |
|
IDLH (Immediate danger)
|
N.D. |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Diazinon (IUPAC name: O,O-Diethyl O-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate, INN - Dimpylate), a colorless to dark brown liquid, is a thiophosphoric acid ester developed in 1952 by Ciba-Geigy, a Swiss chemical company (later Novartis and then Syngenta). It is a nonsystemic organophosphate insecticide formerly used to control cockroaches, silverfish, ants, and fleas in residential, non-food buildings. Diazinon was heavily used during the 1970s and early 1980s for general-purpose gardening use and indoor pest control. A bait form was used to control scavenger wasps in the western U.S. Diazinon is used in flea collars for domestic pets in Australia and New Zealand. Residential uses of diazinon were outlawed in the U.S. in 2004 but it is still approved for agricultural uses. An emergency antidote is atropine.
Diazinon was developed in 1952 by the Swiss company Ciba-Geigy to replace the insecticide DDT. Diazinon became available for mass use in 1955, as DDT production tapered. Before 1970 diazinon had issues with contaminants in the solution. but by the 1970s, alternative purification methods were used to reduce residual materials. After this, diazinon became an all-purpose indoor and outdoor commercial pest control product. In 2004, U.S. residential use of diazinon was outlawed, except for agricultural purposes and cattle ear tags .
The structure of diazinon contains a thiophosphoric ester. The phosphorus center is the reactive site of the chemical. However, no known mechanisms currently exist. A novel mechanism does exist, which proposes that the sulfur is protonated in acidic medium via a hydronium ion which ultimately delivers a hydroxide group to the phosphorus center and can react readily.
The form of diazinon varies widely as it can be in dusts, granules, liquids, concentrates, microencapsulations, wettable powders, and seed dressings. Its appearance varies depending on purity, ranging from a dark brown (industrial grade), to a colorless liquid (pure). Indicative of its functionality of a thiophosphoric ester, the chemical has a pronounced smell similar to that of diethyl ether.
...
Wikipedia
