Dexmethylphenidate
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Focalin, Focalin XR, Attenade |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a603014 |
| Pregnancy category |
|
| Routes of administration |
oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 11 – 52% |
| Protein binding | 30% |
| Metabolism | hepatic |
| Biological half-life | 4 hours |
| Excretion | renal |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
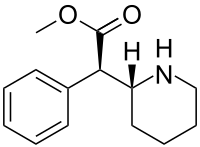
| Formula | C14H19NO2 |
| Molar mass | 233.31 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Dexmethylphenidate (trade names Focalin, Attenade; also known as d-threo-methylphenidate (D-TMP)) is a central nervous system (CNS) stimulant of the phenethylamine and piperidine classes that is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It is the active dextrorotatory enantiomer of methylphenidate.
Dexmethylphenidate is used as a treatment for ADHD, usually along with psychological, educational, behavioral or other forms of treatment. It is proposed that stimulants help ameliorate the symptoms of ADHD by making it easier for the user to concentrate, avoid distraction, and control behavior. Placebo-controlled trials have shown that once-daily dexmethylphenidate XR was effective and generally well tolerated. Improvements in ADHD symptoms in children were significantly greater for dexmethylphenidate XR versus placebo. It also showed greater efficacy than osmotic controlled-release oral delivery system (OROS) methylphenidate over the first half of the laboratory classroom day but assessments late in the day favoured OROS methylphenidate.
Methylphenidate is contraindicated for individuals using monoamine oxidase inhibitors (e.g., phenelzine and tranylcypromine), or individuals with agitation, tics, or glaucoma, or a hypersensitivity to any ingredients contained in methylphenidate pharmaceuticals.
The US FDA gives methylphenidate a pregnancy category of C, and women are advised to only use the drug if the benefits outweigh the potential risks. Not enough animal and human studies have been conducted to conclusively demonstrate an effect of methylphenidate on fetal development. In 2007, empirical literature included 63 cases of prenatal exposure to methylphenidate across three empirical studies.
...
Wikipedia
