Desomorphine
 |
|
 |
|
| Clinical data | |
|---|---|
| Trade names | Permonid |
| Dependence liability |
Physical: Very high Psychological: Very high |
| Addiction liability |
Very high |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
|
|
| Synonyms | Desomorphine, dihydrodesoxymorphine, Permonid |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.006.406 |
| Chemical and physical data | |
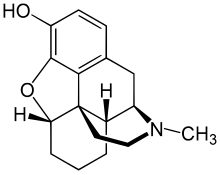
| Formula | C17H21NO2 |
| Molar mass | 271.354 g/mol |
| 3D model (Jmol) | |
|
|
|
|
|
|
|
Desomorphine (dihydrodesoxymorphine, former brand name Permonid, street name krokodil) is a derivative of morphine with powerful, fast-acting opioid effects, such as sedation and analgesia. First synthesized in 1932 and patented in 1934, desomorphine was used in Switzerland under the brand name Permonid and was described as having a fast onset and a short duration of action, with relatively little nausea compared to equivalent doses of morphine. Dose-by-dose it is eight to ten times more potent than morphine.
Desomorphine is derived from morphine where the 6-hydroxyl group and the 7,8 double bond have been reduced. The traditional synthesis of desomorphine starts from α-chlorocodide, which is itself obtained by reacting thionyl chloride with codeine. By catalytic reduction, α-chlorocodide gives dihydrodesoxycodeine, which yields desomorphine on demethylation.
Desomorphine was previously used in Switzerland and Russia for the treatment of severe pain; although for many years up to 1981, when its use was terminated, it was being used to treat a single person in Bern, Switzerland with a rare illness. While desomorphine was found to be faster acting and more effective than morphine for the rapid relief of severe pain, its shorter duration of action and the relatively more severe respiratory depression produced at equianalgesic doses were felt to outweigh any potential advantages.
Desomorphine abuse in Russia attracted international attention in 2010 due to an increase in clandestine production, presumably due to its relatively simple synthesis from codeine available over-the-counter. Abuse of homemade desomorphine was first reported in Siberia in 2003 when Russia started a major crackdown on heroin production and trafficking, but has since spread throughout Russia and the neighboring former Soviet republics.
...
Wikipedia
