Dehydroascorbate
 |
|
| Names | |
|---|---|
|
IUPAC name
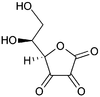
(5R)-5-[(1S)-1,2-dihydroxyethyl]furan-2,3,4(5H)-trione
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.019 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C6H6O6 | |
| Molar mass | 174.11 g·mol−1 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Dehydroascorbic acid (DHA) is an oxidized form of ascorbic acid (vitamin C). It is actively imported into the endoplasmic reticulum of cells via glucose transporters. It is trapped therein by reduction back to ascorbate by glutathione and other thiols. The (free) chemical radical semidehydroascorbic acid (SDA) also belongs to the group of oxidized ascorbic acids.
Although a sodium-dependent transporter for vitamin C exists, it is present mainly in specialized cells, whereas the glucose transporters, the most notable being GLUT1, transport Vitamin C (in its oxidized form, DHA) in most cells, where recycling back to ascorbate generates the necessary enzyme cofactor and intracellular antioxidant, (see Transport to mitochondria).
The structure shown here for DHA is the commonly shown textbook structure. This 1,2,3-tricarbonyl is too electrophilic to survive more than a few milliseconds in aqueous solution, however. The actual structure shown by spectroscopic studies is the result of rapid hemiacetal formation between the 6-OH and the 3-carbonyl groups. Hydration of the 2-carbonyl is also observed. The lifetime of the stabilized species is commonly said to be about 6 minutes under biological conditions. Destruction results from irreversible hydrolysis of the ester bond, with additional degradation reactions following. Crystallization of solutions of DHA gives a pentacyclic dimer structure of indefinite stability. Recycling of ascorbate via active transport of DHA into cells, followed by reduction and reuse, mitigates the inability of humans to synthesize it from glucose.
Vitamin C accumulates in , where most of the free radicals are produced, by entering as DHA through the glucose transporters, GLUT10. Ascorbic acid protects the and .
Vitamin C does not pass from the bloodstream into the brain, although the brain is one of the organs that have the greatest concentration of vitamin C. Instead, DHA is transported through the blood–brain barrier via GLUT1 transporters, and then converted back to ascorbate.
Dehydroascorbic acid has been used as a vitamin C dietary supplement.
As a cosmetic ingredient, dehydroascorbic acid is used to enhance the appearance of the skin. It may be used in a process for permanent waving of hair and in a process for sunless tanning of skin.
...
Wikipedia
