Decavanadate
 |
|
 |
|
| Identifiers | |
|---|---|
| Properties | |
| Na6[V10O28] | |
| Molar mass | 1419.6 g |
| Appearance | orange solid |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Sodium decavanadate describes any member of the family of inorganic compounds with the formula Na6[V10O28](H2O)n. These are sodium salts of the orange-colored decavanadate anion [V10O28]6−. Numerous other decavanadate salts have been isolated and studied since 1956 when it was first characterized.
The preparation of decavanadate is achieved by acidifying an aqueous solution of ortho-vanadate:
The formation of decavanadate is optimized by maintaining a pH range of 4–7. Typical side products include metavanadate, [VO3]−, and hexavanadate, [V6O16]2−, ions.
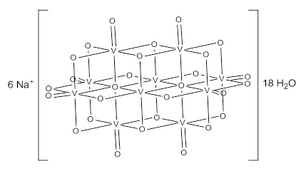
The decavanadate ion consists of 10 fused VO6 octahedra and has D2h symmetry. The structure of Na6[V10O28]·18H2O has been confirmed with X-ray crystallography.
The decavanadate anions contains three sets of equivalent V atoms (see fig. 1). These include two central VO6 octahedra (Vc) and four each peripheral tetragonal-pyramidal VO5 groups (Va and Vb). There are seven unique groups of oxygen atoms (labeled A through G). Two of these (A) bridge to six V centers, four (B) bridge three V centers, fourteen of these (C, D and E) span edges between pairs of V centers, and eight (F and G) are peripheral.
The oxidation state of vanadium in decavanadate is +5.
Aqueous vanadate (V) compounds undergo various self-condensation reactions. Depending on pH, major vanadate anions in solution include VO2(H2O)42+, VO43−, V2O73−, V3O93−, V4O124−, and V10O266−. The anions often reversibly protonate. Decavanadate forms according to this equilibrium:
The structure of the various protonation states of the decavanadate ion has been examined by 51V NMR spectroscopy. Each species gives three signals; with slightly varying chemical shifts around −425, −506, and −523 ppm relative to vanadium oxytrichloride; suggesting that rapid proton exchange occurs resulting in equally symmetric species. The three protonations of decavanadate have been shown to occur at the bridging oxygen centers, indicated as B and C in figure 1.
...
Wikipedia
