D-glucose
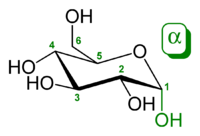 α-D-glucopyranose (chair form).
|
|
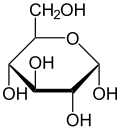
Haworth projection of α-D-glucopyranose
|
|

Fischer projection of D-glucose
|
|
| Names | |
|---|---|
| Pronunciation | /ˈɡluːkoʊz/, /ˈɡluːkoʊs/ |
|
Preferred IUPAC name
D-Glucose
|
|
|
Systematic IUPAC name
(2R,3S,4R,5R)-2,3,4,5,6-Pentahydroxyhexanal
|
|
| Other names
Blood sugar
Dextrose Corn sugar D-Glucose Grape sugar |
|
| Identifiers | |
| 3D model (Jmol) |
Interactive image Interactive image |
| 3DMet | B04623 |
| Abbreviations | Glc |
| 1281604 | |
| ChEBI |
CHEBI:4167 |
| ChEMBL |
ChEMBL1222250 |
| ChemSpider |
5589 |
| EC Number | 200-075-1 |
| 83256 | |
| 4536 | |
| KEGG |
C00031 |
| MeSH | Glucose |
| PubChem | 5793 |
| RTECS number | LZ6600000 |
| UNII |
5SL0G7R0OK |
|
|
|
|
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g·mol−1 |
| Appearance | White powder |
| Density | 1.54 g/cm3 |
| Melting point | α-D-glucose: 146 °C (295 °F; 419 K) β-D-glucose: 150 °C (302 °F; 423 K) |
| 909 g/1 L (25 °C (77 °F)) | |
| -101.5·10−6 cm3/mol | |
| 8.6827 | |
| Thermochemistry | |
| 218.6 J K−1 mol−1 | |
|
Std molar
entropy (S |
209.2 J K−1 mol−1 |
|
Std enthalpy of
formation (ΔfH |
−1271 kJ/mol |
|
Std enthalpy of
combustion (ΔcH |
−2805 kJ/mol |
| Pharmacology | |
| B05CX01 (WHO) V04CA02 (WHO), V06DC01 (WHO) | |
| Hazards | |
| Safety data sheet | ICSC 08655 |
| NFPA 704 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Glucose is a simple sugar with the molecular formula C6H12O6. Glucose circulates in the blood of animals as blood sugar. It is made during photosynthesis from water and carbon dioxide, using energy from sunlight. The reverse of the photosynthesis reaction, which releases this energy, is an important source of power for cellular respiration. Glucose is stored as a polymer, in plants as starch and in animals as glycogen, for times when the organism will need it.
With 6 carbon atoms, it is classed as a hexose, a sub-category of the monosaccharides. D-glucose is one of the 16 aldohexose stereoisomers. The D-isomer, D-glucose, also known as dextrose, occurs widely in nature, but the L-isomer, L-glucose, does not. Glucose can be obtained by hydrolysis of carbohydrates such as milk sugar, cane sugar, maltose, cellulose, glycogen etc. It is commonly commercially manufactured from cornstarch by hydrolysis via pressurized steaming at controlled pH in a jet followed by further enzymatic depolymerization.
In 1747, Andreas Marggraf was the first to isolate glucose. Glucose is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system. The name glucose derives through the French from the Greek γλυκός, which means "sweet," in reference to must, the sweet, first press of grapes in the making of wine. The suffix "-ose" is a chemical classifier, denoting a carbohydrate.
...
Wikipedia

