Cystine knot
| Cystine-knot domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
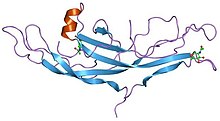
Structure of human chorionic gonadotropin.
|
|||||||||
| Identifiers | |||||||||
| Symbol | Cys_knot | ||||||||
| Pfam | PF00007 | ||||||||
| Pfam clan | CL0079 | ||||||||
| InterPro | IPR006208 | ||||||||
| SCOP | 1hcn | ||||||||
| SUPERFAMILY | 1hcn | ||||||||
|
|||||||||
| Available protein structures: | |
|---|---|
| Pfam | structures |
| PDB | RCSB PDB; PDBe; PDBj |
| PDBsum | structure summary |
A cystine knot is a protein structural motif containing three disulfide bridges (formed from pairs of cysteine residues). The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane substructure. It occurs in many proteins across many species and provides considerable structural stability. There are three types of cystine knot, which differ in the topology of the disulfide bonds:
The growth factor cystine knot (GFCK) was first observed in the structure of Nerve Growth Factor, solved by X-ray crystallography and published in 1991 by Tom Blundell in Nature. All GFCK structures that have been determined are dimeric, but their dimerization modes in different classes are different.
...
Wikipedia
