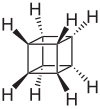
Cubane
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Cubane
|
|||
|
Systematic IUPAC name
Pentacyclo[4.2.0.02,5.03,8.04,7]octane
|
|||
| Identifiers | |||
|
277-10-1 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChEBI |
CHEBI:33014 |
||
| ChemSpider |
119867 |
||
| PubChem | 136090 | ||
|
|||
|
|||
| Properties | |||
| C8H8 | |||
| Molar mass | 104.15 g/mol | ||
| Density | 1.29 g/cm3 | ||
| Melting point | 133.5 °C (272.3 °F; 406.6 K) | ||
| Boiling point | 161.6 °C (322.9 °F; 434.8 K) | ||
| Related compounds | |||
|
Related hydrocarbons
|
Cuneane Dodecahedrane Tetrahedrane Prismane Prismane C8 |
||
|
Related compounds
|
Heptanitrocubane Octanitrocubane Octaazacubane |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Cubane (C8H8) is a synthetic hydrocarbon molecule that consists of eight carbon atoms arranged at the corners of a cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substance, cubane is one of the Platonic hydrocarbons and a member of the prismanes. It was first synthesized in 1964 by Philip Eaton and Thomas Cole. Prior to this work, researchers believed that cubic carbon-based molecules would be too unstable to exist. The cubic shape requires the carbon atoms to adopt an unusually sharp 90° bonding angle, which would be highly strained as compared to the 109.45° angle of a tetrahedral carbon. Once formed, cubane is quite kinetically stable, due to a lack of readily available decomposition paths. It is the simplest hydrocarbon with octahedral symmetry.
Having high energy but kinetic stability makes cubane and its derivative compounds useful for controlled energy storage. For example, octanitrocubane and heptanitrocubane have been studied as high-performance explosives.
These compounds also typically have a very high density for hydrocarbon molecules. The resulting high energy density means a large amount of energy can be stored in a comparably small amount of space, an important consideration for applications in fuel storage and energy transport.
The classic 1964 synthesis starts with the conversion of 2-cyclopentenone to 2-bromocyclopentadienone:
...
Wikipedia