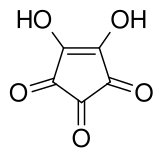
Croconic acid
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
4,5-Dihydroxycyclopent-4-ene-1,2,3-trione
|
|||
| Other names
Crocic acid
|
|||
| Identifiers | |||
|
488-86-8 |
|||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider |
476003 |
||
| ECHA InfoCard | 100.201.686 | ||
| PubChem | 546874 | ||
|
|||
|
|||
| Properties | |||
| C5H2O5 | |||
| Molar mass | 142.07 | ||
| Melting point | > 300 °C (572 °F; 573 K) (decomposes) | ||
| Acidity (pKa) | 0.80, 2.24 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Croconic acid or 4,5-dihydroxycyclopentenetrione is a chemical compound with formula C5H2O5 or (C=O)3(COH)2. It has a cyclopentene backbone with two hydroxyl groups adjacent to the double bond and three ketone groups on the remaining carbon atoms. It is sensitive to light, soluble in water and ethanol and forms yellow crystals that decompose at 212 °C.
The compound is acidic and loses the hydrogen cations H+ from the hydroxyls (pK1 = 0.80 ± 0.08 and pK2 = 2.24 ± 0.01 at 25 °C). The resulting anions, hydrogencroconate C5HO5− and croconate C5O52− are also quite stable. The croconate ion, in particular, is aromatic and symmetric, as the double bond and the negative charges become delocalized over the five CO units. The lithium, sodium and potassium croconates crystallize from water as dihydrates but the orange potassium salt can be dehydrated to form a monohydrate. The croconates of ammonium, rubidium and caesium crystallize in the anhydrous form. Salts of barium, lead, silver, etc. are also known.
Croconic acid also forms ethers such as dimethyl croconate where the hydrogen atom of the hydroxyl group is substituted with an alkyl group.
Croconic acid and potassium croconate dihydrate were discovered by Leopold Gmelin in 1825, who named the compounds from Greek κρόκος meaning "saffron" or "egg yolk". The structure of ammonium croconate was determined by Baenziger et al. in 1964. The structure of K2C5O5 . 2H2O was determined by J. D. Dunitz in 2001
...
Wikipedia


