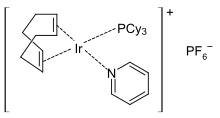
Crabtree's catalyst
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
(SP-4)tris(cyclohexyl)phosphane
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.164.161 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C31H50F6IrNP2 | |
| Molar mass | 804.9026 g/mol |
| Melting point | 150 °C (302 °F; 423 K) (decomposes) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Crabtree's catalyst is an organoiridium compound with the formula [C8H12IrP(C6H11)3C5H5N]PF6. It is a homogeneous catalyst for hydrogenation and hydrogen-transfer reactions, developed by Robert H. Crabtree. This air stable orange solid is available commercially.
The complex has a square planar molecular geometry, as expected for a d8 complex. It is prepared from cyclooctadiene iridium chloride dimer.
Crabtree’s catalyst is effective for the hydrogenations of mono-, di-, tri-, and tetra-substituted substrates. Whereas Wilkinson’s catalyst and the Schrock–Osborn catalyst do not catalyze the hydrogenation of a tetrasubstituted olefin, Crabtree’s catalyst does so to at high turnover frequencies (table).
The catalyst is reactive at room temperature. The reaction is robust without drying solvents or meticulous deoxygenation of the hydrogen. The catalyst is tolerant of weakly basic functional groups such as ester, but not alcohols (see below) or amines. The catalyst is sensitive to proton-bearing impurities.
The catalyst becomes irreversibly deactivated after about ten minutes at room temperature, signaled by appearance of yellow color. One deactivation process involves formation of hydride-bridged dimers.
Besides hydrogenation, the catalyst catalyzes the isomerization and hydroboration of alkenes.
Crabtree's catalyst is used in isotope exchange reactions. More specifically, it catalyzes the direct exchange of a hydrogen atom with its isotopes deuterium and tritium, without the use of an intermediate. It has been shown that isotope exchange with Crabtree’s catalyst is highly regioselective.
The hydrogenation of a terpen-4-ol demonstrates the ability of compounds with directing groups (the –OH group) to undergo diastereoselective hydrogenation. With palladium on carbon in ethanol the product distribution is 20:80 favoring the cis isomer (2B in Scheme 1). The polar side (with the hydroxyl group) interacts with the solvent. This is due to slight haptophilicity, an effect in which a functional group binds to the surface of a heterogeneous catalyst and directs the reaction. In cyclohexane as solvent, the distribution changes to 53:47 because haptophilicity is no long present (there is no directing group on cyclohexane). The distribution changes completely in favor of the cis isomer 2A when Crabtree's catalyst is used in dichloromethane. This selectivity is both predictable and practically useful. Carbonyl groups are also known to direct the hydrogenation by the Crabtree catalyst to be highly regioselective.
...
Wikipedia
