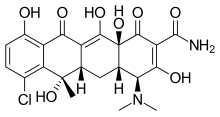
Chlortetracycline
 |
|
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration |
By mouth, IV, topical |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 30% |
| Protein binding | 50 to 55% |
| Metabolism | Hepatic (75%) |
| Biological half-life | 5.6 to 9 hours |
| Excretion | Renal and biliary |
| Identifiers | |
|
|
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | E702 (antibiotics) |
| ECHA InfoCard | 100.000.310 |
| Chemical and physical data | |
| Formula | C22H23ClN2O8 |
| Molar mass | 478.882 g/mol |
| 3D model (Jmol) | |
| Melting point | 168 to 169 °C (334 to 336 °F) |
|
|
|
|
|
|
|
Chlortetracycline (trade name Aureomycin, Lederle) is a tetracycline antibiotic, the first tetracycline to be identified. It was discovered in 1945 by Benjamin Minge Duggar working at Lederle Laboratories under the supervision of Yellapragada Subbarow. Duggar identified the antibiotic as the product of an actinomycete he cultured from a soil sample collected from Sanborn Field at the University of Missouri. The organism was named Streptomyces aureofaciens and the isolated drug, Aureomycin, because of their golden color.
In veterinary medicine, chlortetracycline is commonly used to treat conjunctivitis in cats.
Chlortetracycline may increase the anticoagulant activities of acenocoumarol. The risk or severity of adverse effects can be increased when chlortetracycline is combined with acitretin, adapalene, or alitretinoin. Aluminum phosphate and aluminum hydroxide can cause decreases in the absorption of chlortetracycline resulting in a reduced serum concentration and potentially a decrease in efficacy. The therapeutic efficacy of mecillinam (amdinocillin), amoxicillin, and ampicillin can be decreased when used in combination with chlortetracycline. Chlortetracycline may increase the neuromuscular blocking activities of atracurium besilate.
...
Wikipedia
