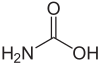
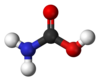
Carbamoyl
|
|
|||
| Names | |||
|---|---|---|---|
|
IUPAC name
Carbamic acid
|
|||
| Other names
Aminomethanoic Acid
|
|||
| Identifiers | |||
|
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
| MeSH | Carbamic+acid | ||
|
PubChem CID
|
|||
|
|||
|
|||
| Properties | |||
| CH3NO2 | |||
| Molar mass | 61.040 g/mol | ||
| Related compounds | |||
|
Related compounds
|
Formamide Dithiocarbamate Carbonic acid Urea Ethyl carbamate Glycine |
||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Carbamic acid is the compound with the formula NH2COOH. The attachment of the acid group to a nitrogen or amine (instead of carbon) distinguishes it from carboxylic acid and an amide. Many derivatives and analogues of carbamic acid are known. They are generally unstable, reverting to the parent amine and carbon dioxide. The deprotonated anion (or conjugate base) of this functional group is a carbamate. Carbamic acid is a planar molecule.
Carbamic acid is an intermediate in the production of urea, which involves the reaction of carbon dioxide and ammonia.
"Carbamoyltransferases" are transferase enzymes classified under EC number 2.1.3.
carbamic acid group
carbamate group
carbamoyl (Cbm) group
carbamoyl chloride group
Carbamic acids are intermediates in the decomposition of carbamate protecting groups; the hydrolysis of an ester bond produces carbamic acid the evolution of carbon dioxide drives the deprotection reaction forward, yielding the unprotected amine.
Carbamates usually refer to esters of carbamic acid. Methyl carbamate is the simplest ester of carbamic acid. Unlike carbamic acids, the esters are stable. They are prepared by reaction of carbamoyl chlorides with alcohols, the addition of alcohols to isocyanates, and the reaction of carbonate esters with ammonia.
Some esters have use as muscle relaxants which bind to the barbiturate site of the GABAA receptor, while others are used as insecticides, for example aldicarb.
...
Wikipedia