Cannabichromene
 |
|
| Names | |
|---|---|
|
IUPAC name
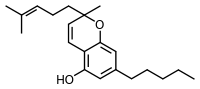
2-Methyl-2-(4-methylpent-3-enyl)-7-pentyl-5-chromenol
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.236.929 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| C21H30O2 | |
| Molar mass | 314.46 g/mol |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Cannabichromene (CBC) is one of the 113 cannabinoids found in the Cannabis plant and therefore can be also described as a - from ancient Greek phyton = "plant". It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol, tetrahydrocannabivarin, cannabidiol, and cannabinol, among others . Evidence has suggested that it may play a role in the anti-inflammatory and anti-viral effects of cannabis, and it may have also antifungal properties.American Cancer Society Cannabichromene may contribute to the overall analgesic effects of medical cannabis. A study done in March 2010 showed that CBC along with cannabidiol and tetrahydrocannabinol have antidepressant effects. Another study showed that CBC helps promote neurogenesis.
CBC is known to interact with many receptors in the brain. It is widely known that the cannabinoids interact with the CB1 and CB2 receptors, but they also interact with others. CBC in particular is known to interact with the TRPV1 and TRPA1 receptors as well, which may result in some of its medicinal properties. Also, in mice, its antiinflammatory activity appears to be modulated by the administration of THC and is independent of the CB2 receptor. This suggests an interplay of the two molecules.
CBC has two stereoisomers. It is not scheduled by the Convention on Psychotropic Substances. CBC is non-psychotropic. Its boiling point is 220 degrees Celsius, 428 degrees Fahrenheit.
Within the Cannabis plant, CBC occurs mainly as cannabichromenic acid (CBCA, 2-COOH-CBC, CBC-COOH). Geranyl pyrophosphate and olivetolic acid combine to produce cannabigerolic acid (a key intermediate for multiple cannabinoids), which is cyclized by the enzyme CBCA synthease to give CBCA. Over time, or when heated, CBCA is decarboxylated, producing CBC. See biosynthetic scheme below the chemical data table.
...
Wikipedia
