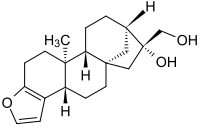
Cafestol
 |
|
| Names | |
|---|---|
|
IUPAC name
(3bS,5aS,7R,8R,10aR,10bS)-3b,4,5,6,7,8,9,10,10a,10b,11,12-Dodecahydro-7-hydroxy-10b-methyl-5a,8-methano-5aH-cyclohepta[5,6]naphtho[2,1-b]furan-7-methanol
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| KEGG | |
|
PubChem CID
|
|
| UNII | |
|
|
|
|
| Properties | |
| C20H28O3 | |
| Molar mass | 316.44 g·mol−1 |
| Melting point | 158 to 162 °C (316 to 324 °F; 431 to 435 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
| Infobox references | |
Cafestol is a diterpene molecule present in coffee.
A typical bean of Coffea arabica contains about 0.4-0.7% cafestol by weight. Cafestol is present in highest quantity in unfiltered coffee drinks such as French press coffee or Turkish coffee/Greek coffee. In filtered coffee drinks such as drip brewed coffee, it is present in only negligible amounts.
Studies have shown that regular consumption of boiled coffee increases serum cholesterol by 8% in men and 10% in women. For those drinking filter coffee, the effect was only significant for women.
Cafestol has also shown anticarcinogenic properties in rats. Cafestol may act as an agonist ligand for the nuclear receptor farnesoid X receptor and pregnane X receptor, blocking cholesterol homeostasis. Cafestol also has neuroprotective effects in a Drosophila fruit fly model of Parkinson's disease.
...
Wikipedia
