CR gas
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
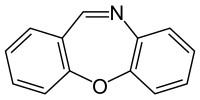
Dibenzo[b,f][1,4]oxazepine
|
|
| Identifiers | |
|
257-07-8 |
|
| 3D model (Jmol) | Interactive image |
| ChEMBL |
ChEMBL1085100 |
| ChemSpider |
8858 |
| ECHA InfoCard | 100.114.990 |
| 6472 | |
| PubChem | 9213 |
|
|
|
|
| Properties | |
| C13H9NO | |
| Molar mass | 195.22 g/mol |
| Density | 1.160±0.10 g/cm3 |
| Melting point | 73 °C (163 °F; 346 K) |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
CR gas or dibenzoxazepine (also referred to as DBO), or its chemical name dibenz[b,f][1,4]oxazepine, is an incapacitating agent and a lachrymatory agent. CR was developed by the British Ministry of Defence as a riot control agent in the late 1950s and early 1960s. A report from the Porton Down laboratories described exposure as 'like being thrown blindfolded into a bed of stinging nettles', and it earned the nickname "firegas".
CR is a pale yellow crystalline solid with a pepper-like odor. It is slightly soluble in water and does not degrade in it. CR is usually presented as a microparticulate solid, in the form of suspension in a propylene glycol-based liquid. Contrary to its common name, it is not a gas but a solid at room temperature.
The dibenz[b,f][1,4]oxazepine moiety is present in the typical antipsychotic drug loxapine, but, unlike CR, loxapine is not reactive and is not an irritant. CR was first synthesised in 1962.
CR can be delivered either as an aerosol or a solution in water, making it able to be used in water cannons, smoke grenades, or handheld spray cans. For smoke it is usually fired in canisters (LACR) that heat up, producing an aerosol cloud at a steady rate.
CR gas is a lachrymatory agent (LA), exerting its effects through activation of the TRPA1 channel. Its effects are approximately 6 to 10 times more powerful than those of CS gas. CR causes intense skin irritation, in particular around moist areas; blepharospasm, causing temporary blindness; and coughing, gasping for breath, and panic. It is capable of causing immediate incapacitation. It is a suspected carcinogen. It is toxic, but less so than CS gas, by ingestion and exposure. However, it can be lethal in large quantities. In a poorly ventilated space, an individual may inhale a lethal dose within minutes. Death is caused by asphyxiation and pulmonary edema.
...
Wikipedia
