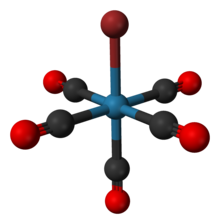
Bromopentacarbonylrhenium
 |
|
| Names | |
|---|---|
|
IUPAC name
Bromidopentacarbonylrhenium
|
|
| Identifiers | |
|
3D model (JSmol)
|
|
| ECHA InfoCard | 100.034.607 |
| EC Number | 238-084-8 |
|
PubChem CID
|
|
|
|
|
|
| Properties | |
| Re(CO)5Br | |
| Molar mass | 406.16 g/mol |
| Appearance | colorless |
| Melting point | sublimes 85-90 °C (0.2 mm Hg) |
| Solubility in chlorocarbons | soluble |
| Hazards | |
| GHS pictograms |
 
|
| GHS signal word | Damger |
| H301, H311, H315, H319, H331, H335 | |
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P305+351+338, P311, P312, P321, P322, P330, P332+313, P337+313, P361, P362, P363, P403+233, P405, P501 | |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
Bromopentacarbonylrhenium(I) is an inorganic compound of rhenium, commonly used for the syntheses of other rhenium complexes.
Bromopentacarbonylrhenium(I) is commercially available. It is also easily and inexpensively synthesized by the oxidation of dirhenium decacarbonyl with bromine:
It was first prepared by the "reductive carbonylation" of rhenium(III) bromide:
Copper(I) bromide is a byproduct.
Bromopentacarbonylrhenium(I) is a useful intermediate to other rhenium complexes. For example, it reacts with zinc and acetic acid to give pentacarbonylhydridorhenium (ReH(CO)5).
It also reacts with tetraethylammonium bromide in diglyme to give [NEt4]2[ReBr3(CO)3)], an important precursor to compounds containing the rhenium tricarbonyl fragment.
Refluxing bromopentacarbonylrhenium(I) in water also provides access to the rhenium tricarbonyl fragment:
This route avoids the formation of the tetraethylammonium bromide byproduct when the tetraethylammonium complex is used. This is desirable because the tetraethylammonium bromide is often difficult to remove from reaction mixtures.
...
Wikipedia
