Dirhenium decacarbonyl
 |
|
 |
|
| Names | |
|---|---|
|
IUPAC name
bis(pentacarbonylrhenium)(Re—Re)
|
|
| Other names
Rhenium carbonyl; rhenium pentacarbonyl
|
|
| Identifiers | |
|
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.034.714 |
|
|
|
|
| Properties | |
| Re2(CO)10 | |
| Molar mass | 652.52 g/mol |
| Melting point | 170C (decomposes) |
| Hazards | |
|
EU classification (DSD)
|
Harmful (Xn) |
| R-phrases | R20 |
| S-phrases | S36 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|
|
|
|
| Infobox references | |
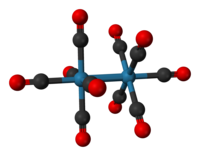
Dirhenium decacarbonyl is the inorganic compound with the chemical formula Re2(CO)10 . Commercially available, it is used as a starting point for the synthesis of many rhenium carbonyl complexes. It was first reported in 1941 by Walter Hieber, who prepared it by reductive carbonylation of rhenium. The compound consists of a pair of square pyramidal Re(CO)5 units joined via a Re-Re bond, which produces a homoleptic carbonyl complex.
In the 1930s Robert Mond developed methods which used increased pressure and temperature to produce various forms of metal carbonyl . A prominent scientist of the twentieth century, Walter Hieber was crucial to the further development of specifically the dirhenium decacarbonyl. Initial efforts produced mononuclear metal complexes, but upon further evaluation, Hieber discovered that by using Re2O7 as a starting material with no solvent, a dirhenium complex could be achieved producing a Re-Re interaction.
The crystal structure of Re2(CO)10 is relatively well known. The compound consists of a pair of square pyramidal Re(CO)5 units linked by a Re-Re bond. There are two different conformations that can occur: staggered and eclipsed. The eclipsed conformation occurs about 30% of the time, producing a D4hpoint group, but the staggered form, with point group D4d, is more stable. The Re-Re bond length was experimentally found to be 3.04Å.
The Re atom exists in a slightly distorted octahedral configuration with the C axial-Re-C equatorial angle equal to 88°. The mean Re-C bond length of 2.01 Å is the same for the axial and equatorial positions. The mean C-O distance is 1.16 Å.
This compound has a broad IR absorption band at 1800 cm−1 region can be assigned to two components centered at 1780 and 1830 cm−1, resulting from CO adsorption. The remaining nine CO groups in Re2(CO)10 give the complex IR absorption in the 1950–2150 cm−1 region. Free Re2(CO)10 (point symmetry D4d ) has a CO stretch representation of 2A1+E2 + E3+ 2B2 +E1, where 2B2 + E1 are IR active. For an axially perturbed (C4v) Re2(CO)10 molecule, the CO stretch representation was found to be 2E+B1+B2+3A1, where the IR active modes are 2E+3A1.
...
Wikipedia
