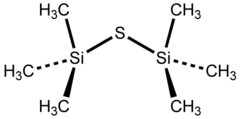
Bis(trimethylsilyl)sulfide
 |
|||
|
|
|||
| Names | |||
|---|---|---|---|
|
Preferred IUPAC name
Bis(trimethylsilyl) sulfide
|
|||
|
Systematic IUPAC name
Trimethyl[(trimethylsilyl)sulfanyl]silane
|
|||
| Other names
Hexamethyldisilathiane
|
|||
| Identifiers | |||
|
3D model (Jmol)
|
|||
| 1698358 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.020.184 | ||
| EC Number | 222-201-4 | ||
|
PubChem CID
|
|||
| UN number | 1993 | ||
|
|||
|
|||
| Properties | |||
| C6H18SSi2 | |||
| Molar mass | 178.44 g·mol−1 | ||
| Appearance | colourless liquid with foul odor | ||
| Density | 0.846 g cm−3 | ||
| Boiling point | 163 °C (325 °F; 436 K) | ||
| hydrolyzes | |||
| Solubility in other solvents | ethers such as THF and arenes such as toluene |
||
|
Refractive index (nD)
|
1.4586 | ||
| Structure | |||
| 1.85 D | |||
| Hazards | |||
| Main hazards | Toxic | ||
| Safety data sheet | "External MSDS" | ||
| GHS pictograms |
 
|
||
| GHS signal word | Danger | ||
| H226, H331, H311, H301 | |||
| P261, P280, P301+310, P311 | |||
| R-phrases | R10-R23/24/25 | ||
| S-phrases | S36/37/39-S38-S45 | ||
| NFPA 704 | |||
| Related compounds | |||
|
Related compounds
|
B2S3, SiS2 | ||
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
|
|||
|
|
|||
| Infobox references | |||
Bis(trimethylsilyl) sulfide is the chemical compound with the formula ((CH3)3Si)2S. Often abbreviated (tms)2S, this colourless, vile-smelling liquid is a useful aprotic source of “S2−“ in chemical synthesis.
The reagent is prepared by treating trimethylsilyl chloride with anhydrous sodium sulfide:
((CH3)3Si)2S must be protected from air because it hydrolyzes readily:
Bis(trimethylsilyl)sulfide is a reagent for the conversion of metal oxides and chlorides into the corresponding sulfides. This transformation exploits the affinity of silicon(IV) for oxygen and halides. An idealized reaction is:
In a similar way, it has been used in the conversion of aldehydes and ketones to the corresponding thiones.
((CH3)3Si)2S reacts exothermically with water, releasing toxic H2S.
...
Wikipedia