Biomimetic synthesis
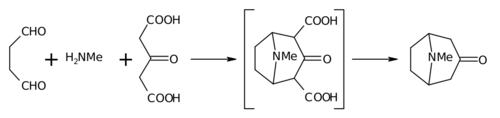
Biomimetic synthesis is an area of organic chemical synthesis that is specifically biologically inspired, so-named in 1917 by the English organic chemist and Nobel laureate Sir Robert Robinson. The term encompasses both the testing of a "biogenetic hypothesis" (conjectured course of a biosynthesis in nature) through execution of a series of reactions designed to parallel the proposed biosynthesis, as well as programs of study where a synthetic reaction or reactions aimed at a desired synthetic goal are designed to mimic a one or more known enzymic transformations of an established biosynthetic pathway. The earliest generally cited example of a biomimetic synthesis is Robinson's organic synthesis of the alkaloid tropinone.
A more recent example is E.J. Corey's carbenium-mediated cyclization of an engineered linear polyene to provide a tetracyclic steroid ring system, which built upon studies of cationic cyclizations of linear polyenes by the Albert Eschenmoser and Gilbert Stork, and the extensive studies of the W.S. Johnson to define the requirements to initiate and terminate the cyclization, and to stabilize the cationic carbenium group during the cyclization (as nature accomplishes via enzymes during biosynthesis of steroids such as cholesterol). In relation to the second definition, synthetic organic or inorganic catalysts applied to accomplish a chemical transformation accomplished in nature by a biocatalyst (e.g., a purely proteinaceous catalyst, a metal or other cofactor bound to an enzyme, or a ribozyme) can be said to be accomplishing a biomimetic synthesis, where design and characterization of such catalytic systems has been termed biomimetic chemistry.
...
Wikipedia